| Activity |
|---|
| Catalytic type | Cysteine |
| Peplist | Included in the Peplist with identifier PL00119 |
| NC-IUBMB | Subclass 3.4 (Peptidases) >> Sub-subclass 3.4.22 (Cysteine endopeptidases) >> Peptidase 3.4.22.49
|
| Enzymology | BRENDA database |
| Proteolytic events | CutDB database (12 cleavages) |
| Activity status | human: active (Peters & Nasmyth, 2004)
mouse: active (Terret et al., 2003)
|
| Physiology | In cell division, prior to anaphase, the pairs of sister chromatids are bound together by a protein called cohesin. Separin is the endopeptidase that can cleave cohesin (at arginine residues). The activity of separin is inhibited by securin, but this is destroyed at the start of anaphase, allowing separin to cleave cohesin, and the chromosomes to separate towards the poles of the spindle. This is a vital function in the division of eukaryotic cells (Nasmyth et al., 2000). It has also been shown that the cleavage of Rec8 by separin during meiosis of yeast is necessary for the resolution of chiasmata and the disjunction of homologous chromosomes (Buonomo et al., 2000). A mutation in separase is responsible for cell cycle defects in zebrafish (Shepard et al., 2007). |
| Pathways |
KEGG | Cell cycle - yeast |
|
KEGG | Meiosis - yeast |
|
Other databases
| PANTHER | http://www.pantherdb.org/panther/familyList.do?searchType=basic&fieldName=all&listType=6&fieldValue=PTHR12792:SF0 |
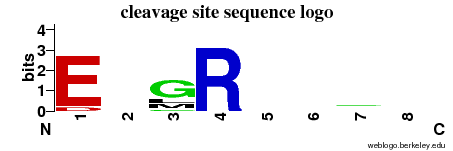
| Cleavage site specificity |
Explanations of how to interpret the
following cleavage site sequence logo and specificity matrix can be found here. |
|---|
| Cleavage pattern | E/-/Glm/R -/-/-/- (based on 12 cleavages) -/-/-/- (based on 12 cleavages) |

 -/-/-/- (based on 12 cleavages)
-/-/-/- (based on 12 cleavages)