 |
PDBsum entry 2gri

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Viral protein
|
PDB id
|
|

|

|
2gri
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Viral protein
|
 |
|
Title:
|
 |
Nmr structure of the sars-cov non-structural protein nsp3a
|
|
Structure:
|
 |
Nsp3. Chain: a. Engineered: yes
|
|
Source:
|
 |
Sars coronavirus. Organism_taxid: 227859. Expressed in: escherichia coli. Expression_system_taxid: 562.
|
|
NMR struc:
|
 |
20 models
|
 |
|
Authors:
|
 |
P.Serrano,M.S.Almeida,M.A.Johnson,T.Herrmann,K.S.Saikatendu,J.Joseph, V.Subramanian,B.W.Neuman,M.J.Buchmeier,R.C.Stevens,P.Kuhn, K.Wuthrich,Joint Center For Structural Genomics (Jcsg)
|
|
Key ref:
|
 |
P.Serrano
et al.
(2007).
Nuclear magnetic resonance structure of the N-terminal domain of nonstructural protein 3 from the severe acute respiratory syndrome coronavirus.
J Virol,
81,
12049-12060.
PubMed id:
|
 |
|
Date:
|
 |
|
24-Apr-06
|
Release date:
|
19-Dec-06
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P0C6X7
(R1AB_CVHSA) -
Replicase polyprotein 1ab from Severe acute respiratory syndrome coronavirus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
7073 a.a.
112 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.2.1.1.-
- ?????
|
|
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.2.1.1.56
- mRNA (guanine-N(7))-methyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 5'-end (5'-triphosphoguanosine)-ribonucleoside in mRNA + S-adenosyl-L- methionine = a 5'-end (N(7)-methyl 5'-triphosphoguanosine)-ribonucleoside in mRNA + S-adenosyl-L-homocysteine
|
 |
 |
 |
 |
 |
5'-end (5'-triphosphoguanosine)-ribonucleoside in mRNA
|
+
|
 S-adenosyl-L- methionine
S-adenosyl-L- methionine
|
=
|
5'-end (N(7)-methyl 5'-triphosphoguanosine)-ribonucleoside in mRNA
|
+
|
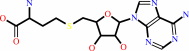 S-adenosyl-L-homocysteine
S-adenosyl-L-homocysteine
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 4:
|
 |
E.C.2.1.1.57
- methyltransferase cap1.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 5'-end (N(7)-methyl 5'-triphosphoguanosine)-ribonucleoside in mRNA + S-adenosyl-L-methionine = a 5'-end (N(7)-methyl 5'-triphosphoguanosine)- (2'-O-methyl-ribonucleoside) in mRNA + S-adenosyl-L-homocysteine + H+
|
 |
 |
 |
 |
 |
5'-end (N(7)-methyl 5'-triphosphoguanosine)-ribonucleoside in mRNA
|
+
|
 S-adenosyl-L-methionine
S-adenosyl-L-methionine
|
=
|
5'-end (N(7)-methyl 5'-triphosphoguanosine)- (2'-O-methyl-ribonucleoside) in mRNA
|
+
|
 S-adenosyl-L-homocysteine
S-adenosyl-L-homocysteine
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 5:
|
 |
E.C.2.7.7.48
- RNA-directed Rna polymerase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
RNA(n) + a ribonucleoside 5'-triphosphate = RNA(n+1) + diphosphate
|
 |
 |
 |
 |
 |
 RNA(n)
RNA(n)
|
+
|
ribonucleoside 5'-triphosphate
|
=
|
RNA(n+1)
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 6:
|
 |
E.C.2.7.7.50
- mRNA guanylyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 5'-end diphospho-ribonucleoside in mRNA + GTP + H+ = a 5'-end (5'-triphosphoguanosine)-ribonucleoside in mRNA + diphosphate
|
 |
 |
 |
 |
 |
5'-end diphospho-ribonucleoside in mRNA
|
+
|
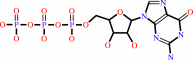 GTP
GTP
|
+
|
H(+)
|
=
|
5'-end (5'-triphosphoguanosine)-ribonucleoside in mRNA
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 7:
|
 |
E.C.3.1.13.-
- ?????
|
|
 |
 |
 |
 |
 |
Enzyme class 8:
|
 |
E.C.3.4.19.12
- ubiquitinyl hydrolase 1.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
Thiol-dependent hydrolysis of ester, thiolester, amide, peptide and isopeptide bonds formed by the C-terminal Gly of ubiquitin (a 76-residue protein attached to proteins as an intracellular targeting signal).
|
 |
 |
 |
 |
 |
Enzyme class 9:
|
 |
E.C.3.4.22.-
- ?????
|
|
 |
 |
 |
 |
 |
Enzyme class 10:
|
 |
E.C.3.4.22.69
- Sars coronavirus main proteinase.
|
|
 |
 |
 |
 |
 |
Enzyme class 11:
|
 |
E.C.3.6.4.12
- Dna helicase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
ATP + H2O = ADP + phosphate + H+
|
 |
 |
 |
 |
 |
 ATP
ATP
|
+
|
H2O
|
=
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 12:
|
 |
E.C.3.6.4.13
- Rna helicase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
ATP + H2O = ADP + phosphate + H+
|
 |
 |
 |
 |
 |
 ATP
ATP
|
+
|
H2O
|
=
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 13:
|
 |
E.C.4.6.1.-
- ?????
|
|
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
J Virol
81:12049-12060
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
Nuclear magnetic resonance structure of the N-terminal domain of nonstructural protein 3 from the severe acute respiratory syndrome coronavirus.
|
|
P.Serrano,
M.A.Johnson,
M.S.Almeida,
R.Horst,
T.Herrmann,
J.S.Joseph,
B.W.Neuman,
V.Subramanian,
K.S.Saikatendu,
M.J.Buchmeier,
R.C.Stevens,
P.Kuhn,
K.Wüthrich.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
This paper describes the structure determination of nsp3a, the N-terminal domain
of the severe acute respiratory syndrome coronavirus (SARS-CoV) nonstructural
protein 3. nsp3a exhibits a ubiquitin-like globular fold of residues 1 to 112
and a flexibly extended glutamic acid-rich domain of residues 113 to 183. In
addition to the four beta-strands and two alpha-helices that are common to
ubiquitin-like folds, the globular domain of nsp3a contains two short helices
representing a feature that has not previously been observed in these proteins.
Nuclear magnetic resonance chemical shift perturbations showed that these unique
structural elements are involved in interactions with single-stranded RNA.
Structural similarities with proteins involved in various cell-signaling
pathways indicate possible roles of nsp3a in viral infection and persistence.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
K.R.Hurst,
R.Ye,
S.J.Goebel,
P.Jayaraman,
and
P.S.Masters
(2010).
An interaction between the nucleocapsid protein and a component of the replicase-transcriptase complex is crucial for the infectivity of coronavirus genomic RNA.
|
| |
J Virol,
84,
10276-10288.
|
 |
|
|
|
|
 |
A.Chatterjee,
M.A.Johnson,
P.Serrano,
B.Pedrini,
J.S.Joseph,
B.W.Neuman,
K.Saikatendu,
M.J.Buchmeier,
P.Kuhn,
and
K.Wüthrich
(2009).
Nuclear magnetic resonance structure shows that the severe acute respiratory syndrome coronavirus-unique domain contains a macrodomain fold.
|
| |
J Virol,
83,
1823-1836.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.Tan,
C.Vonrhein,
O.S.Smart,
G.Bricogne,
M.Bollati,
Y.Kusov,
G.Hansen,
J.R.Mesters,
C.L.Schmidt,
and
R.Hilgenfeld
(2009).
The SARS-Unique Domain (SUD) of SARS Coronavirus Contains Two Macrodomains That Bind G-Quadruplexes.
|
| |
PLoS Pathog,
5,
e1000428.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
P.Serrano,
M.A.Johnson,
A.Chatterjee,
B.W.Neuman,
J.S.Joseph,
M.J.Buchmeier,
P.Kuhn,
and
K.Wüthrich
(2009).
Nuclear magnetic resonance structure of the nucleic acid-binding domain of severe acute respiratory syndrome coronavirus nonstructural protein 3.
|
| |
J Virol,
83,
12998-13008.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.Perlman,
and
J.Netland
(2009).
Coronaviruses post-SARS: update on replication and pathogenesis.
|
| |
Nat Rev Microbiol,
7,
439-450.
|
 |
|
|
|
|
 |
B.W.Neuman,
J.S.Joseph,
K.S.Saikatendu,
P.Serrano,
A.Chatterjee,
M.A.Johnson,
L.Liao,
J.P.Klaus,
J.R.Yates,
K.Wüthrich,
R.C.Stevens,
M.J.Buchmeier,
and
P.Kuhn
(2008).
Proteomics analysis unravels the functional repertoire of coronavirus nonstructural protein 3.
|
| |
J Virol,
82,
5279-5294.
|
 |
|
|
|
|
 |
P.Serrano,
M.A.Johnson,
A.Chatterjee,
B.Pedrini,
and
K.Wüthrich
(2008).
NMR assignment of the nonstructural protein nsp3(1066-1181) from SARS-CoV.
|
| |
Biomol NMR Assign,
2,
135-138.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

