
|

|

|
|
 |
Contents |
 |
|
|

|

|

|

|

|

|

|

 655 a.a.
655 a.a.
|
 |

|

|

|

|

|

|

|

 239 a.a.
239 a.a.
|
 |

|

|

|

|

|

|

|

 254 a.a.
254 a.a.
|
 |

|

|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
Respiratory complex ii-like fumarate reductase from wolinella succinogenes
|
|
Structure:
|
 |
Fumarate reductase flavoprotein subunit. Chain: a, d. Other_details: 8-alpha-[-n-epsilon-histidyl] covalent bond between flavin adenine dinucleotide (fad) and his 43. Fumarate reductase iron-sulfur protein. Chain: b, e. Fumarate reductase cytochrome b subunit. Chain: c, f. Other_details: haem axial ligands - his 44, his 93, his 143, his 182
|
|
Source:
|
 |
Wolinella succinogenes. Organism_taxid: 844. Organism_taxid: 844
|
|
Biol. unit:
|
 |
Hexamer (from
)
|
|
Resolution:
|
 |
|
2.33Å
|
R-factor:
|
0.213
|
R-free:
|
0.223
|
|
|
Authors:
|
 |
C.R.D.Lancaster,A.Kroeger,M.Auer,H.Michel
|
Key ref:

|
 |
C.R.Lancaster
et al.
(1999).
Structure of fumarate reductase from Wolinella succinogenes at 2.2 A resolution.
Nature,
402,
377-385.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
25-Aug-99
|
Release date:
|
29-Nov-99
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P17412
(FRDA_WOLSU) -
Fumarate reductase flavoprotein subunit from Wolinella succinogenes (strain ATCC 29543 / DSM 1740 / CCUG 13145 / JCM 31913 / LMG 7466 / NCTC 11488 / FDC 602W)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
656 a.a.
655 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B, D, E:
E.C.1.3.5.1
- succinate dehydrogenase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Citric acid cycle
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a quinone + succinate = fumarate + a quinol
|
 |
 |
 |
 |
 |
 quinone
quinone
|
+
|
succinate
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
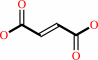 fumarate
fumarate
|
+
|
quinol
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FAD; Iron-sulfur
|
 |
 |
 |
 |
 |
FAD
Bound ligand (Het Group name =
FAD)
corresponds exactly
|
 Iron-sulfur
Iron-sulfur
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Nature
402:377-385
(1999)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of fumarate reductase from Wolinella succinogenes at 2.2 A resolution.
|
|
C.R.Lancaster,
A.Kröger,
M.Auer,
H.Michel.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Fumarate reductase couples the reduction of fumarate to succinate to the
oxidation of quinol to quinone, in a reaction opposite to that catalysed by the
related complex II of the respiratory chain (succinate dehydrogenase). Here we
describe the crystal structure at 2.2 A resolution of the three protein subunits
containing fumarate reductase from the anaerobic bacterium Wolinella
succinogenes. Subunit A contains the site of fumarate reduction and a covalently
bound flavin adenine dinucleotide prosthetic group. Subunit B contains three
iron-sulphur centres. The menaquinol-oxidizing subunit C consists of five
membrane-spanning, primarily helical segments and binds two haem b molecules. On
the basis of the structure, we propose a pathway of electron transfer from the
dihaem cytochrome b to the site of fumarate reduction and a mechanism of
fumarate reduction. The relative orientations of the soluble and
membrane-embedded subunits of succinate:quinone oxidoreductases appear to be
unique.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1 Representative parts of the experimental
electron-density maps for crystal form A calculated with the
MIRAS phases after density modification and phase extension to
2.2 ? resolution. C, N, O, P and S atoms are shown in grey,
blue, red, light green and green, respectively; haem iron
centres are shown in orange. Contour levels are 1.0  (green)
and 9.0 (green)
and 9.0  (red)
above the mean density of the map. Figs 1-4 and 6 were prepared
with a version of Molscript46 modified by R. Esnouf for colour
ramping47 and map drawing48 capabilities. a, b, The two haem b
molecules (b[P] in the top half; b[D] in the bottom half of each
panel) and the side chains of some neighbouring residues in the
transmembrane region. c, The covalently bound FAD prosthetic
group. (red)
above the mean density of the map. Figs 1-4 and 6 were prepared
with a version of Molscript46 modified by R. Esnouf for colour
ramping47 and map drawing48 capabilities. a, b, The two haem b
molecules (b[P] in the top half; b[D] in the bottom half of each
panel) and the side chains of some neighbouring residues in the
transmembrane region. c, The covalently bound FAD prosthetic
group.
|
 |
Figure 2.
Figure 2 The three-dimensional structure of fumarate
reductase. a, The fumarate reductase dimer viewed parallel to
the membrane. The polypeptide backbones of the two A subunits
are shown in blue and light blue, those of the two B subunits in
red and pink, and those of the C subunits in green and yellow.
Subunit A contains a covalently bound FAD. Subunit B contains
three iron-sulphur clusters (Fe[2]S[2], Fe[4]S[4] and
Fe[3]S[4]). The membrane-embedded subunit C contains two haem b
molecules. b, View of the transmembrane helices of the subunit C
dimer along the membrane normal from the cytoplasmic side. One
monomer is colour-coded from blue (N terminus) to yellow (C
terminus), the other from yellow (N terminus) to red (C
terminus)). The transmembrane helices are labelled I, II, IV, V
and VI (ref. 22). Secondary structures were assigned using
DSSP49. Figure rendered with Raster3D^50.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Macmillan Publishers Ltd:
Nature
(1999,
402,
377-385)
copyright 1999.
|
|
| |
Figures were
selected
by the author.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
K.Illergård,
A.Kauko,
and
A.Elofsson
(2011).
Why are polar residues within the membrane core evolutionary conserved?
|
| |
Proteins,
79,
79-91.
|
 |
|
|
|
|
 |
T.Kurihara
(2011).
A mechanistic analysis of enzymatic degradation of organohalogen compounds.
|
| |
Biosci Biotechnol Biochem,
75,
189-198.
|
 |
|
|
|
|
 |
C.M.Paquete,
and
R.O.Louro
(2010).
Molecular details of multielectron transfer: the case of multiheme cytochromes from metal respiring organisms.
|
| |
Dalton Trans,
39,
4259-4266.
|
 |
|
|
|
|
 |
K.R.Vinothkumar,
and
R.Henderson
(2010).
Structures of membrane proteins.
|
| |
Q Rev Biophys,
43,
65.
|
 |
|
|
|
|
 |
L.J.Smith,
A.Kahraman,
and
J.M.Thornton
(2010).
Heme proteins--diversity in structural characteristics, function, and folding.
|
| |
Proteins,
78,
2349-2368.
|
 |
|
|
|
|
 |
N.V.Azarkina,
and
A.A.Konstantinov
(2010).
Energization of Bacillus subtilis membrane vesicles increases catalytic activity of succinate:menaquinone oxidoreductase.
|
| |
Biochemistry (Mosc),
75,
50-62.
|
 |
|
|
|
|
 |
W.Q.Chen,
A.Salmazo,
M.Myllykoski,
B.Sjöblom,
M.Bidlingmaier,
A.Pollak,
P.Baumgärtel,
K.Djinovic-Carugo,
P.Kursula,
and
G.Lubec
(2010).
Purification of recombinant growth hormone by clear native gels for conformational analyses: preservation of conformation and receptor binding.
|
| |
Amino Acids,
39,
859-869.
|
 |
|
|
|
|
 |
H.D.Juhnke,
H.Hiltscher,
H.R.Nasiri,
H.Schwalbe,
and
C.R.Lancaster
(2009).
Production, characterization and determination of the real catalytic properties of the putative 'succinate dehydrogenase' from Wolinella succinogenes.
|
| |
Mol Microbiol,
71,
1088-1101.
|
 |
|
|
|
|
 |
J.Ruprecht,
V.Yankovskaya,
E.Maklashina,
S.Iwata,
and
G.Cecchini
(2009).
Structure of Escherichia coli succinate:quinone oxidoreductase with an occupied and empty quinone-binding site.
|
| |
J Biol Chem,
284,
29836-29846.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
D.S.Lee,
P.Nioche,
M.Hamberg,
and
C.S.Raman
(2008).
Structural insights into the evolutionary paths of oxylipin biosynthetic enzymes.
|
| |
Nature,
455,
363-368.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
E.A.Berry,
and
F.A.Walker
(2008).
Bis-histidine-coordinated hemes in four-helix bundles: how the geometry of the bundle controls the axial imidazole plane orientations in transmembrane cytochromes of mitochondrial complexes II and III and related proteins.
|
| |
J Biol Inorg Chem,
13,
481-498.
|
 |
|
|
|
|
 |
J.Meyer
(2008).
Iron-sulfur protein folds, iron-sulfur chemistry, and evolution.
|
| |
J Biol Inorg Chem,
13,
157-170.
|
 |
|
|
|
|
 |
M.Jormakka,
K.Yokoyama,
T.Yano,
M.Tamakoshi,
S.Akimoto,
T.Shimamura,
P.Curmi,
and
S.Iwata
(2008).
Molecular mechanism of energy conservation in polysulfide respiration.
|
| |
Nat Struct Mol Biol,
15,
730-737.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.M.Tomasiak,
E.Maklashina,
G.Cecchini,
and
T.M.Iverson
(2008).
A threonine on the active site loop controls transition state formation in Escherichia coli respiratory complex II.
|
| |
J Biol Chem,
283,
15460-15468.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
W.Liu,
C.E.Rogge,
G.F.da Silva,
V.P.Shinkarev,
A.L.Tsai,
Y.Kamensky,
G.Palmer,
and
R.J.Kulmacz
(2008).
His92 and His110 selectively affect different heme centers of adrenal cytochrome b(561).
|
| |
Biochim Biophys Acta,
1777,
1218-1228.
|
 |
|
|
|
|
 |
J.D.Otero-Cruz,
C.A.Báez-Pagán,
I.M.Caraballo-González,
and
J.A.Lasalde-Dominicci
(2007).
Tryptophan-scanning mutagenesis in the alphaM3 transmembrane domain of the muscle-type acetylcholine receptor. A spring model revealed.
|
| |
J Biol Chem,
282,
9162-9171.
|
 |
|
|
|
|
 |
X.Liang,
D.J.Campopiano,
and
P.J.Sadler
(2007).
Metals in membranes.
|
| |
Chem Soc Rev,
36,
968-992.
|
 |
|
|
|
|
 |
A.Y.Mulkidjanian,
and
D.A.Cherepanov
(2006).
Probing biological interfaces by tracing proton passage across them.
|
| |
Photochem Photobiol Sci,
5,
577-587.
|
 |
|
|
|
|
 |
E.Maklashina,
P.Hellwig,
R.A.Rothery,
V.Kotlyar,
Y.Sher,
J.H.Weiner,
and
G.Cecchini
(2006).
Differences in protonation of ubiquinone and menaquinone in fumarate reductase from Escherichia coli.
|
| |
J Biol Chem,
281,
26655-26664.
|
 |
|
|
|
|
 |
E.Maklashina,
T.M.Iverson,
Y.Sher,
V.Kotlyar,
J.Andréll,
O.Mirza,
J.M.Hudson,
F.A.Armstrong,
R.A.Rothery,
J.H.Weiner,
and
G.Cecchini
(2006).
Fumarate reductase and succinate oxidase activity of Escherichia coli complex II homologs are perturbed differently by mutation of the flavin binding domain.
|
| |
J Biol Chem,
281,
11357-11365.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.E.Butler,
R.H.Glaven,
A.Esteve-Núñez,
C.Núñez,
E.S.Shelobolina,
D.R.Bond,
and
D.R.Lovley
(2006).
Genetic characterization of a single bifunctional enzyme for fumarate reduction and succinate oxidation in Geobacter sulfurreducens and engineering of fumarate reduction in Geobacter metallireducens.
|
| |
J Bacteriol,
188,
450-455.
|
 |
|
|
|
|
 |
J.Zhang,
F.E.Frerman,
and
J.J.Kim
(2006).
Structure of electron transfer flavoprotein-ubiquinone oxidoreductase and electron transfer to the mitochondrial ubiquinone pool.
|
| |
Proc Natl Acad Sci U S A,
103,
16212-16217.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.L.Pankhurst,
C.G.Mowat,
E.L.Rothery,
J.M.Hudson,
A.K.Jones,
C.S.Miles,
M.D.Walkinshaw,
F.A.Armstrong,
G.A.Reid,
and
S.K.Chapman
(2006).
A proton delivery pathway in the soluble fumarate reductase from Shewanella frigidimarina.
|
| |
J Biol Chem,
281,
20589-20597.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.S.Huang,
G.Sun,
D.Cobessi,
A.C.Wang,
J.T.Shen,
E.Y.Tung,
V.E.Anderson,
and
E.A.Berry
(2006).
3-nitropropionic acid is a suicide inhibitor of mitochondrial respiration that, upon oxidation by complex II, forms a covalent adduct with a catalytic base arginine in the active site of the enzyme.
|
| |
J Biol Chem,
281,
5965-5972.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.S.Huang,
J.T.Shen,
A.C.Wang,
and
E.A.Berry
(2006).
Crystallographic studies of the binding of ligands to the dicarboxylate site of Complex II, and the identity of the ligand in the "oxaloacetate-inhibited" state.
|
| |
Biochim Biophys Acta,
1757,
1073-1083.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.G.Madej,
H.R.Nasiri,
N.S.Hilgendorff,
H.Schwalbe,
and
C.R.Lancaster
(2006).
Evidence for transmembrane proton transfer in a dihaem-containing membrane protein complex.
|
| |
EMBO J,
25,
4963-4970.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.Horsefield,
V.Yankovskaya,
G.Sexton,
W.Whittingham,
K.Shiomi,
S.Omura,
B.Byrne,
G.Cecchini,
and
S.Iwata
(2006).
Structural and computational analysis of the quinone-binding site of complex II (succinate-ubiquinone oxidoreductase): a mechanism of electron transfer and proton conduction during ubiquinone reduction.
|
| |
J Biol Chem,
281,
7309-7316.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.Teschner,
L.Yatsunyk,
V.Schünemann,
H.Paulsen,
H.Winkler,
C.Hu,
W.R.Scheidt,
F.A.Walker,
and
A.X.Trautwein
(2006).
Models of the membrane-bound cytochromes: mössbauer spectra of crystalline low-spin ferriheme complexes having axial ligand plane dihedral angles ranging from 0 degree to 90 degrees.
|
| |
J Am Chem Soc,
128,
1379-1389.
|
 |
|
|
|
|
 |
A.Kurata,
T.Kurihara,
H.Kamachi,
and
N.Esaki
(2005).
2-Haloacrylate reductase, a novel enzyme of the medium chain dehydrogenase/reductase superfamily that catalyzes the reduction of a carbon-carbon double bond of unsaturated organohalogen compounds.
|
| |
J Biol Chem,
280,
20286-20291.
|
 |
|
|
|
|
 |
C.R.Lancaster,
U.S.Sauer,
R.Gross,
A.H.Haas,
J.Graf,
H.Schwalbe,
W.Mäntele,
J.Simon,
and
M.G.Madej
(2005).
Experimental support for the "E pathway hypothesis" of coupled transmembrane e- and H+ transfer in dihemic quinol:fumarate reductase.
|
| |
Proc Natl Acad Sci U S A,
102,
18860-18865.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
F.Sun,
X.Huo,
Y.Zhai,
A.Wang,
J.Xu,
D.Su,
M.Bartlam,
and
Z.Rao
(2005).
Crystal structure of mitochondrial respiratory membrane protein complex II.
|
| |
Cell,
121,
1043-1057.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.A.Rothery,
A.M.Seime,
A.M.Spiers,
E.Maklashina,
I.Schröder,
R.P.Gunsalus,
G.Cecchini,
and
J.H.Weiner
(2005).
Defining the Q-site of Escherichia coli fumarate reductase by site-directed mutagenesis, fluorescence quench titrations and EPR spectroscopy.
|
| |
FEBS J,
272,
313-326.
|
 |
|
|
|
|
 |
S.T.Ohnishi,
T.Ohnishi,
S.Muranaka,
H.Fujita,
H.Kimura,
K.Uemura,
K.Yoshida,
and
K.Utsumi
(2005).
A possible site of superoxide generation in the complex I segment of rat heart mitochondria.
|
| |
J Bioenerg Biomembr,
37,
1.
|
 |
|
|
|
|
 |
T.Kurokawa,
and
J.Sakamoto
(2005).
Purification and characterization of succinate:menaquinone oxidoreductase from Corynebacterium glutamicum.
|
| |
Arch Microbiol,
183,
317-324.
|
 |
|
|
|
|
 |
W.Iwasaki,
H.Miyatake,
and
K.Miki
(2005).
Crystal structure of the small form of glucose-inhibited division protein A from Thermus thermophilus HB8.
|
| |
Proteins,
61,
1121-1126.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Andreeva,
D.Howorth,
S.E.Brenner,
T.J.Hubbard,
C.Chothia,
and
A.G.Murzin
(2004).
SCOP database in 2004: refinements integrate structure and sequence family data.
|
| |
Nucleic Acids Res,
32,
D226-D229.
|
 |
|
|
|
|
 |
A.H.Haas,
and
C.R.Lancaster
(2004).
Calculated coupling of transmembrane electron and proton transfer in dihemic quinol:fumarate reductase.
|
| |
Biophys J,
87,
4298-4315.
|
 |
|
|
|
|
 |
K.S.Oyedotun,
and
B.D.Lemire
(2004).
The quaternary structure of the Saccharomyces cerevisiae succinate dehydrogenase. Homology modeling, cofactor docking, and molecular dynamics simulation studies.
|
| |
J Biol Chem,
279,
9424-9431.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.S.Oyedotun,
P.F.Yau,
and
B.D.Lemire
(2004).
Identification of the heme axial ligands in the cytochrome b562 of the Saccharomyces cerevisiae succinate dehydrogenase.
|
| |
J Biol Chem,
279,
9432-9439.
|
 |
|
|
|
|
 |
M.Gimpelev,
L.R.Forrest,
D.Murray,
and
B.Honig
(2004).
Helical packing patterns in membrane and soluble proteins.
|
| |
Biophys J,
87,
4075-4086.
|
 |
|
|
|
|
 |
R.Das,
and
M.Gerstein
(2004).
A method using active-site sequence conservation to find functional shifts in protein families: application to the enzymes of central metabolism, leading to the identification of an anomalous isocitrate dehydrogenase in pathogens.
|
| |
Proteins,
55,
455-463.
|
 |
|
|
|
|
 |
R.Gross,
R.Pisa,
M.Sänger,
C.R.Lancaster,
and
J.Simon
(2004).
Characterization of the menaquinone reduction site in the diheme cytochrome b membrane anchor of Wolinella succinogenes NiFe-hydrogenase.
|
| |
J Biol Chem,
279,
274-281.
|
 |
|
|
|
|
 |
T.J.Stevens,
K.Mizuguchi,
and
I.T.Arkin
(2004).
Distinct protein interfaces in transmembrane domains suggest an in vivo folding model.
|
| |
Protein Sci,
13,
3028-3037.
|
 |
|
|
|
|
 |
D.A.Cherepanov,
B.A.Feniouk,
W.Junge,
and
A.Y.Mulkidjanian
(2003).
Low dielectric permittivity of water at the membrane interface: effect on the energy coupling mechanism in biological membranes.
|
| |
Biophys J,
85,
1307-1316.
|
 |
|
|
|
|
 |
G.Cecchini
(2003).
Function and structure of complex II of the respiratory chain.
|
| |
Annu Rev Biochem,
72,
77.
|
 |
|
|
|
|
 |
H.Miyadera,
A.Hiraishi,
H.Miyoshi,
K.Sakamoto,
R.Mineki,
K.Murayama,
K.V.Nagashima,
K.Matsuura,
S.Kojima,
and
K.Kita
(2003).
Complex II from phototrophic purple bacterium Rhodoferax fermentans displays rhodoquinol-fumarate reductase activity.
|
| |
Eur J Biochem,
270,
1863-1874.
|
 |
|
|
|
|
 |
H.Miyadera,
K.Shiomi,
H.Ui,
Y.Yamaguchi,
R.Masuma,
H.Tomoda,
H.Miyoshi,
A.Osanai,
K.Kita,
and
S.Omura
(2003).
Atpenins, potent and specific inhibitors of mitochondrial complex II (succinate-ubiquinone oxidoreductase).
|
| |
Proc Natl Acad Sci U S A,
100,
473-477.
|
 |
|
|
|
|
 |
J.Guo,
and
B.D.Lemire
(2003).
The ubiquinone-binding site of the Saccharomyces cerevisiae succinate-ubiquinone oxidoreductase is a source of superoxide.
|
| |
J Biol Chem,
278,
47629-47635.
|
 |
|
|
|
|
 |
L.Hederstedt
(2003).
Structural biology. Complex II is complex too.
|
| |
Science,
299,
671-672.
|
 |
|
|
|
|
 |
M.G.Bertero,
R.A.Rothery,
M.Palak,
C.Hou,
D.Lim,
F.Blasco,
J.H.Weiner,
and
N.C.Strynadka
(2003).
Insights into the respiratory electron transfer pathway from the structure of nitrate reductase A.
|
| |
Nat Struct Biol,
10,
681-687.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.H.Hefti,
J.Vervoort,
and
W.J.van Berkel
(2003).
Deflavination and reconstitution of flavoproteins.
|
| |
Eur J Biochem,
270,
4227-4242.
|
 |
|
|
|
|
 |
M.J.Lemieux,
J.Song,
M.J.Kim,
Y.Huang,
A.Villa,
M.Auer,
X.D.Li,
and
D.N.Wang
(2003).
Three-dimensional crystallization of the Escherichia coli glycerol-3-phosphate transporter: a member of the major facilitator superfamily.
|
| |
Protein Sci,
12,
2748-2756.
|
 |
|
|
|
|
 |
M.Jormakka,
B.Byrne,
and
S.Iwata
(2003).
Formate dehydrogenase--a versatile enzyme in changing environments.
|
| |
Curr Opin Struct Biol,
13,
418-423.
|
 |
|
|
|
|
 |
V.Yankovskaya,
R.Horsefield,
S.Törnroth,
C.Luna-Chavez,
H.Miyoshi,
C.Léger,
B.Byrne,
G.Cecchini,
and
S.Iwata
(2003).
Architecture of succinate dehydrogenase and reactive oxygen species generation.
|
| |
Science,
299,
700-704.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
W.Martin,
and
M.J.Russell
(2003).
On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells.
|
| |
Philos Trans R Soc Lond B Biol Sci,
358,
59.
|
 |
|
|
|
|
 |
Z.Li,
J.E.Shokes,
A.Kounosu,
T.Imai,
T.Iwasaki,
and
R.A.Scott
(2003).
X-ray absorption spectroscopic analysis of reductive [2Fe-2S] cluster degradation in hyperthermophilic archaeal succinate:caldariellaquinone oxidoreductase subunits.
|
| |
Biochemistry,
42,
15003-15008.
|
 |
|
|
|
|
 |
A.Brigé,
D.Leys,
T.E.Meyer,
M.A.Cusanovich,
and
J.J.Van Beeumen
(2002).
The 1.25 A resolution structure of the diheme NapB subunit of soluble nitrate reductase reveals a novel cytochrome c fold with a stacked heme arrangement.
|
| |
Biochemistry,
41,
4827-4836.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.G.Tielens,
C.Rotte,
J.J.van Hellemond,
and
W.Martin
(2002).
Mitochondria as we don't know them.
|
| |
Trends Biochem Sci,
27,
564-572.
|
 |
|
|
|
|
 |
B.Byrne,
and
S.Iwata
(2002).
Membrane protein complexes.
|
| |
Curr Opin Struct Biol,
12,
239-243.
|
 |
|
|
|
|
 |
C.Schnarrenberger,
and
W.Martin
(2002).
Evolution of the enzymes of the citric acid cycle and the glyoxylate cycle of higher plants. A case study of endosymbiotic gene transfer.
|
| |
Eur J Biochem,
269,
868-883.
|
 |
|
|
|
|
 |
D.Richardson,
and
G.Sawers
(2002).
Structural biology. PMF through the redox loop.
|
| |
Science,
295,
1842-1843.
|
 |
|
|
|
|
 |
G.Fritz,
A.Roth,
A.Schiffer,
T.Büchert,
G.Bourenkov,
H.D.Bartunik,
H.Huber,
K.O.Stetter,
P.M.Kroneck,
and
U.Ermler
(2002).
Structure of adenylylsulfate reductase from the hyperthermophilic Archaeoglobus fulgidus at 1.6-A resolution.
|
| |
Proc Natl Acad Sci U S A,
99,
1836-1841.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.Simon
(2002).
Enzymology and bioenergetics of respiratory nitrite ammonification.
|
| |
FEMS Microbiol Rev,
26,
285-309.
|
 |
|
|
|
|
 |
L.Adamian,
and
J.Liang
(2002).
Interhelical hydrogen bonds and spatial motifs in membrane proteins: polar clamps and serine zippers.
|
| |
Proteins,
47,
209-218.
|
 |
|
|
|
|
 |
M.J.Sellars,
S.J.Hall,
and
D.J.Kelly
(2002).
Growth of Campylobacter jejuni supported by respiration of fumarate, nitrate, nitrite, trimethylamine-N-oxide, or dimethyl sulfoxide requires oxygen.
|
| |
J Bacteriol,
184,
4187-4196.
|
 |
|
|
|
|
 |
M.Jormakka,
S.Törnroth,
B.Byrne,
and
S.Iwata
(2002).
Molecular basis of proton motive force generation: structure of formate dehydrogenase-N.
|
| |
Science,
295,
1863-1868.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
N.Azarkina,
and
A.A.Konstantinov
(2002).
Stimulation of menaquinone-dependent electron transfer in the respiratory chain of Bacillus subtilis by membrane energization.
|
| |
J Bacteriol,
184,
5339-5347.
|
 |
|
|
|
|
 |
O.Hucke,
R.Schmid,
and
A.Labahn
(2002).
Exploring the primary electron acceptor (QA)-site of the bacterial reaction center from Rhodobacter sphaeroides. Binding mode of vitamin K derivatives.
|
| |
Eur J Biochem,
269,
1096-1108.
|
 |
|
|
|
|
 |
R.H.Spencer,
and
D.C.Rees
(2002).
The alpha-helix and the organization and gating of channels.
|
| |
Annu Rev Biophys Biomol Struct,
31,
207-233.
|
 |
|
|
|
|
 |
R.T.Bossi,
A.Negri,
G.Tedeschi,
and
A.Mattevi
(2002).
Structure of FAD-bound L-aspartate oxidase: insight into substrate specificity and catalysis.
|
| |
Biochemistry,
41,
3018-3024.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Biel,
J.Simon,
R.Gross,
T.Ruiz,
M.Ruitenberg,
and
A.Kröger
(2002).
Reconstitution of coupled fumarate respiration in liposomes by incorporating the electron transport enzymes isolated from Wolinella succinogenes.
|
| |
Eur J Biochem,
269,
1974-1983.
|
 |
|
|
|
|
 |
T.Iwasaki,
A.Kounosu,
M.Aoshima,
D.Ohmori,
T.Imai,
A.Urushiyama,
N.J.Cosper,
and
R.A.Scott
(2002).
Novel [2Fe-2S]-type redox center C in SdhC of archaeal respiratory complex II from Sulfolobus tokodaii strain 7.
|
| |
J Biol Chem,
277,
39642-39648.
|
 |
|
|
|
|
 |
T.M.Iverson,
C.Luna-Chavez,
L.R.Croal,
G.Cecchini,
and
D.C.Rees
(2002).
Crystallographic studies of the Escherichia coli quinol-fumarate reductase with inhibitors bound to the quinol-binding site.
|
| |
J Biol Chem,
277,
16124-16130.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
W.Dietrich,
and
O.Klimmek
(2002).
The function of methyl-menaquinone-6 and polysulfide reductase membrane anchor (PsrC) in polysulfide respiration of Wolinella succinogenes.
|
| |
Eur J Biochem,
269,
1086-1095.
|
 |
|
|
|
|
 |
Z.Ge
(2002).
Potential of fumarate reductase as a novel therapeutic target in Helicobacter pylori infection.
|
| |
Expert Opin Ther Targets,
6,
135-146.
|
 |
|
|
|
|
 |
A.I.Tsapin,
I.Vandenberghe,
K.H.Nealson,
J.H.Scott,
T.E.Meyer,
M.A.Cusanovich,
E.Harada,
T.Kaizu,
H.Akutsu,
D.Leys,
and
J.J.Van Beeumen
(2001).
Identification of a small tetraheme cytochrome c and a flavocytochrome c as two of the principal soluble cytochromes c in Shewanella oneidensis strain MR1.
|
| |
Appl Environ Microbiol,
67,
3236-3244.
|
 |
|
|
|
|
 |
B.E.Schultz,
and
S.I.Chan
(2001).
Structures and proton-pumping strategies of mitochondrial respiratory enzymes.
|
| |
Annu Rev Biophys Biomol Struct,
30,
23-65.
|
 |
|
|
|
|
 |
C.R.Lancaster,
R.Gross,
and
J.Simon
(2001).
A third crystal form of Wolinella succinogenes quinol:fumarate reductase reveals domain closure at the site of fumarate reduction.
|
| |
Eur J Biochem,
268,
1820-1827.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
D.E.Edmondson,
and
P.Newton-Vinson
(2001).
The covalent FAD of monoamine oxidase: structural and functional role and mechanism of the flavinylation reaction.
|
| |
Antioxid Redox Signal,
3,
789-806.
|
 |
|
|
|
|
 |
G.Tedeschi,
S.Ronchi,
T.Simonic,
C.Treu,
A.Mattevi,
and
A.Negri
(2001).
Probing the active site of L-aspartate oxidase by site-directed mutagenesis: role of basic residues in fumarate reduction.
|
| |
Biochemistry,
40,
4738-4744.
|
 |
|
|
|
|
 |
I.Ubarretxena-Belandia,
and
D.M.Engelman
(2001).
Helical membrane proteins: diversity of functions in the context of simple architecture.
|
| |
Curr Opin Struct Biol,
11,
370-376.
|
 |
|
|
|
|
 |
M.Schnorpfeil,
I.G.Janausch,
S.Biel,
A.Kröger,
and
G.Unden
(2001).
Generation of a proton potential by succinate dehydrogenase of Bacillus subtilis functioning as a fumarate reductase.
|
| |
Eur J Biochem,
268,
3069-3074.
|
 |
|
|
|
|
 |
R.J.Bienstock,
and
J.C.Barrett
(2001).
KAI1, a prostate metastasis suppressor: prediction of solvated structure and interactions with binding partners; integrins, cadherins, and cell-surface receptor proteins.
|
| |
Mol Carcinog,
32,
139-153.
|
 |
|
|
|
|
 |
A.W.Munro,
P.Taylor,
and
M.D.Walkinshaw
(2000).
Structures of redox enzymes.
|
| |
Curr Opin Biotechnol,
11,
369-376.
|
 |
|
|
|
|
 |
C.Binda,
R.T.Bossi,
S.Wakatsuki,
S.Arzt,
A.Coda,
B.Curti,
M.A.Vanoni,
and
A.Mattevi
(2000).
Cross-talk and ammonia channeling between active centers in the unexpected domain arrangement of glutamate synthase.
|
| |
Structure,
8,
1299-1308.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.R.Lancaster,
and
A.Kröger
(2000).
Succinate: quinone oxidoreductases: new insights from X-ray crystal structures.
|
| |
Biochim Biophys Acta,
1459,
422-431.
|
 |
|
|
|
|
 |
C.R.Lancaster,
R.Gorss,
A.Haas,
M.Ritter,
W.Mäntele,
J.Simon,
and
A.Kröger
(2000).
Essential role of Glu-C66 for menaquinol oxidation indicates transmembrane electrochemical potential generation by Wolinella succinogenes fumarate reductase.
|
| |
Proc Natl Acad Sci U S A,
97,
13051-13056.
|
 |
|
|
|
|
 |
G.A.Reid,
C.S.Miles,
R.K.Moysey,
K.L.Pankhurst,
and
S.K.Chapman
(2000).
Catalysis in fumarate reductase.
|
| |
Biochim Biophys Acta,
1459,
310-315.
|
 |
|
|
|
|
 |
J.L.Popot,
and
D.M.Engelman
(2000).
Helical membrane protein folding, stability, and evolution.
|
| |
Annu Rev Biochem,
69,
881-922.
|
 |
|
|
|
|
 |
M.K.Doherty,
S.L.Pealing,
C.S.Miles,
R.Moysey,
P.Taylor,
M.D.Walkinshaw,
G.A.Reid,
and
S.K.Chapman
(2000).
Identification of the active site acid/base catalyst in a bacterial fumarate reductase: a kinetic and crystallographic study.
|
| |
Biochemistry,
39,
10695-10701.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.Matsson,
D.Tolstoy,
R.Aasa,
and
L.Hederstedt
(2000).
The distal heme center in Bacillus subtilis succinate:quinone reductase is crucial for electron transfer to menaquinone.
|
| |
Biochemistry,
39,
8617-8624.
|
 |
|
|
|
|
 |
M.le Maire,
P.Champeil,
and
J.V.Moller
(2000).
Interaction of membrane proteins and lipids with solubilizing detergents.
|
| |
Biochim Biophys Acta,
1508,
86.
|
 |
|
|
|
|
 |
R.Camba,
and
F.A.Armstrong
(2000).
Investigations of the oxidative disassembly of Fe-S clusters in Clostridium pasteurianum 8Fe ferredoxin using pulsed-protein-film voltammetry.
|
| |
Biochemistry,
39,
10587-10598.
|
 |
|
|
|
|
 |
R.Ullmann,
R.Gross,
J.Simon,
G.Unden,
and
A.Kröger
(2000).
Transport of C(4)-dicarboxylates in Wolinella succinogenes.
|
| |
J Bacteriol,
182,
5757-5764.
|
 |
|
|
|
|
 |
T.M.Iverson,
C.Luna-Chavez,
I.Schröder,
G.Cecchini,
and
D.C.Rees
(2000).
Analyzing your complexes: structure of the quinol-fumarate reductase respiratory complex.
|
| |
Curr Opin Struct Biol,
10,
448-455.
|
 |
|
|
|
|
 |
T.Ohnishi,
C.C.Moser,
C.C.Page,
P.L.Dutton,
and
T.Yano
(2000).
Simple redox-linked proton-transfer design: new insights from structures of quinol-fumarate reductase.
|
| |
Structure,
8,
R23-R32.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |
|

