 |
PDBsum entry 2bs3

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
2bs3
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|

|

|

|

|

|

 656 a.a.
656 a.a.
|
 |

|

|

|

|

|

|

|

 239 a.a.
239 a.a.
|
 |

|

|

|

|

|

|

|

 255 a.a.
255 a.a.
|
 |

|

|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
Glu c180 -> gln variant quinol:fumarate reductase from wolinella succinogenes
|
|
Structure:
|
 |
Quinol-fumarate reductase flavoprotein subunit a. Chain: a, d. Other_details: fad covalently bound to his a43 by an 8-alpha-(n- epsilon-histidyl) bond. Quinol-fumarate reductase iron-sulfur subunit b. Chain: b, e. Quinol-fumarate reductase diheme cytochrome b subunit c. Chain: c, f. Ec: 1.3.99.1
|
|
Source:
|
 |
Wolinella succinogenes. Organism_taxid: 844. Organism_taxid: 844
|
|
Biol. unit:
|
 |
Hexamer (from PDB file)
|
|
Resolution:
|
 |
|
2.19Å
|
R-factor:
|
0.183
|
R-free:
|
0.198
|
|
|
Authors:
|
 |
C.R.D.Lancaster
|
Key ref:

|
 |
C.R.Lancaster
et al.
(2005).
Experimental support for the "E pathway hypothesis" of coupled transmembrane e- and H+ transfer in dihemic quinol:fumarate reductase.
Proc Natl Acad Sci U S A,
102,
18860-18865.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
14-May-05
|
Release date:
|
13-Dec-05
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P17412
(FRDA_WOLSU) -
Fumarate reductase flavoprotein subunit from Wolinella succinogenes (strain ATCC 29543 / DSM 1740 / CCUG 13145 / JCM 31913 / LMG 7466 / NCTC 11488 / FDC 602W)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
656 a.a.
656 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
Chains A, B, D, E:
E.C.1.3.5.1
- succinate dehydrogenase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Citric acid cycle
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a quinone + succinate = fumarate + a quinol
|
 |
 |
 |
 |
 |
 quinone
quinone
|
+
|
succinate
Bound ligand (Het Group name = )
matches with 61.54% similarity
|
=
|
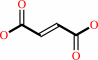 fumarate
fumarate
|
+
|
quinol
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FAD; Iron-sulfur
|
 |
 |
 |
 |
 |
FAD
Bound ligand (Het Group name =
FAD)
corresponds exactly
|
 Iron-sulfur
Iron-sulfur
|
|
 |
 |
Enzyme class 3:
|
 |
Chains C, F:
E.C.1.3.99.1
- Deleted entry.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
Succinate + acceptor = fumarate + reduced acceptor
|
 |
 |
 |
 |
 |
 Succinate
Succinate
|
+
|
acceptor
|
=
|
 fumarate
fumarate
|
+
|
reduced acceptor
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FAD
|
 |
 |
 |
 |
 |
 FAD
FAD
|
|
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Proc Natl Acad Sci U S A
102:18860-18865
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Experimental support for the "E pathway hypothesis" of coupled transmembrane e- and H+ transfer in dihemic quinol:fumarate reductase.
|
|
C.R.Lancaster,
U.S.Sauer,
R.Gross,
A.H.Haas,
J.Graf,
H.Schwalbe,
W.Mäntele,
J.Simon,
M.G.Madej.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Reconciliation of apparently contradictory experimental results obtained on the
quinol:fumarate reductase, a diheme-containing respiratory membrane protein
complex from Wolinella succinogenes, was previously obtained by the proposal of
the so-called "E pathway hypothesis." According to this hypothesis,
transmembrane electron transfer via the heme groups is strictly coupled to
cotransfer of protons via a transiently established pathway thought to contain
the side chain of residue Glu-C180 as the most prominent component. Here we
demonstrate that, after replacement of Glu-C180 with Gln or Ile by site-directed
mutagenesis, the resulting mutants are unable to grow on fumarate, and the
membrane-bound variant enzymes lack quinol oxidation activity. Upon
solubilization, however, the purified enzymes display approximately 1/10 of the
specific quinol oxidation activity of the wild-type enzyme and unchanged quinol
Michaelis constants, K(m). The refined x-ray crystal structures at 2.19 A and
2.76 A resolution, respectively, rule out major structural changes to account
for these experimental observations. Changes in the oxidation-reduction heme
midpoint potential allow the conclusion that deprotonation of Glu-C180 in the
wild-type enzyme facilitates the reoxidation of the reduced high-potential heme.
Comparison of solvent isotope effects indicates that a rate-limiting proton
transfer step in the wild-type enzyme is lost in the Glu-C180 --> Gln variant.
The results provide experimental evidence for the validity of the E pathway
hypothesis and for a crucial functional role of Glu-C180.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Fig. 1. Electron and proton transfer in fumarate
respiration (a) and W. succinogenes QFR (b and c). Positive and
negative sides of the membrane are the periplasm and the
cytoplasm, respectively. Figs. 1, 2, and 3 were prepared with a
version of MOLSCRIPT (39) modified for color ramping (40) and
map drawing (41) capabilities. (a) The key enzymes involved in
fumarate respiration are indicated. (b) Hypothetical
transmembrane electrochemical potential generation as suggested
by the essential role of Glu-C66 for menaquinol oxidation by W.
succinogenes QFR (16). The prosthetic groups of the W.
succinogenes QFR dimer are displayed (coordinate set 1QLA, ref.
14). Distances between prosthetic groups are edge-to-edge
distances in Å as defined by Page et al. (42). Also
indicated are the side chain of Glu-C66 and a model of
menaquinol (MKH[2]) binding. The position of bound fumarate
(Fum) is taken from PDB ID code 1QLB [PDB]
(14). (c) Hypothetical cotransfer of one H+ per electron across
the membrane (E pathway hypothesis). The two protons that are
liberated upon oxidation of menaquinol (MKH[2]) are released to
the periplasm (bottom) via the residue Glu-C66. In compensation,
coupled to electron transfer via the two heme groups, protons
are transferred from the periplasm via the ring C propionate of
the distal heme b[D] and the residue Glu-C180 to the cytoplasm
(top), where they replace those protons that are bound during
fumarate reduction. In the oxidized state of the enzyme, the E
pathway is blocked.
|
 |
Figure 4.
Fig. 4. The coupling of electron and proton flow in
anaerobic (a and b) and aerobic SQRs respiration (c and d),
respectively. Positive and negative sides of the membrane are
described for Fig. 1. (a and b) Electroneutral reactions as
catalyzed by diheme-containing QFR from W. succinogenes (a) and
the hemeless QFR from E. coli. (b) MK and MKH[2], menaquinone
and menaquinol, respectively. (c) Utilization of a transmembrane
electrochemical potential as possibly catalyzed by
diheme-containing SQR enzymes. (d) Electroneutral reaction as
catalyzed by monoheme-containing SQR enzymes (complex II). Q and
QH[2], ubiquinone and ubiquinol, respectively.
|
 |
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
E.Guccione,
A.Hitchcock,
S.J.Hall,
F.Mulholland,
N.Shearer,
A.H.van Vliet,
and
D.J.Kelly
(2010).
Reduction of fumarate, mesaconate and crotonate by Mfr, a novel oxygen-regulated periplasmic reductase in Campylobacter jejuni.
|
| |
Environ Microbiol,
12,
576-591.
|
 |
|
|
|
|
 |
N.V.Azarkina,
and
A.A.Konstantinov
(2010).
Energization of Bacillus subtilis membrane vesicles increases catalytic activity of succinate:menaquinone oxidoreductase.
|
| |
Biochemistry (Mosc),
75,
50-62.
|
 |
|
|
|
|
 |
H.D.Juhnke,
H.Hiltscher,
H.R.Nasiri,
H.Schwalbe,
and
C.R.Lancaster
(2009).
Production, characterization and determination of the real catalytic properties of the putative 'succinate dehydrogenase' from Wolinella succinogenes.
|
| |
Mol Microbiol,
71,
1088-1101.
|
 |
|
|
|
|
 |
E.A.Berry,
and
F.A.Walker
(2008).
Bis-histidine-coordinated hemes in four-helix bundles: how the geometry of the bundle controls the axial imidazole plane orientations in transmembrane cytochromes of mitochondrial complexes II and III and related proteins.
|
| |
J Biol Inorg Chem,
13,
481-498.
|
 |
|
|
|
|
 |
S.Nath
(2008).
The new unified theory of ATP synthesis/hydrolysis and muscle contraction, its manifold fundamental consequences and mechanistic implications and its applications in health and disease.
|
| |
Int J Mol Sci,
9,
1784-1840.
|
 |
|
|
|
|
 |
M.G.Madej,
H.R.Nasiri,
N.S.Hilgendorff,
H.Schwalbe,
and
C.R.Lancaster
(2006).
Evidence for transmembrane proton transfer in a dihaem-containing membrane protein complex.
|
| |
EMBO J,
25,
4963-4970.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |
| |

