
|

|

|
|
 |
Contents |
 |
|
|

|

|

|

|

|

|

|

 577 a.a.
577 a.a.
|
 |

|

|

|

|

|

|

|

 243 a.a.
243 a.a.
|
 |

|

|

|

|

|

|

|

 130 a.a.
130 a.a.
|
 |

|

|

|

|

|

|

|

 119 a.a.
119 a.a.
|
 |

|

|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
E. Coli quinol-fumarate reductase with bound inhibitor hqno
|
|
Structure:
|
 |
Fumarate reductase flavoprotein. Chain: a, m. Engineered: yes. Fumarate reductase iron-sulfur protein. Chain: b, n. Engineered: yes. Fumarate reductase 15 kda hydrophobic protein. Chain: c, o. Engineered: yes.
|
|
Source:
|
 |
Escherichia coli. Organism_taxid: 562. Expressed in: escherichia coli. Expression_system_taxid: 562.
|
|
Biol. unit:
|
 |
Octamer (from
)
|
|
Resolution:
|
 |
|
2.70Å
|
R-factor:
|
0.231
|
R-free:
|
0.280
|
|
|
Authors:
|
 |
T.M.Iverson,C.Luna-Chavez,L.R.Croal,G.Cecchini,D.C.Rees
|
Key ref:

|
 |
T.M.Iverson
et al.
(2002).
Crystallographic studies of the Escherichia coli quinol-fumarate reductase with inhibitors bound to the quinol-binding site.
J Biol Chem,
277,
16124-16130.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
19-Nov-01
|
Release date:
|
13-Mar-02
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P00363
(FRDA_ECOLI) -
Fumarate reductase flavoprotein subunit from Escherichia coli (strain K12)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
602 a.a.
577 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P0AC47
(FRDB_ECOLI) -
Fumarate reductase iron-sulfur subunit from Escherichia coli (strain K12)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
244 a.a.
243 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B, M, N:
E.C.1.3.5.1
- succinate dehydrogenase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Citric acid cycle
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a quinone + succinate = fumarate + a quinol
|
 |
 |
 |
 |
 |
quinone
Bound ligand (Het Group name = )
matches with 88.89% similarity
|
+
|
 succinate
succinate
|
=
|
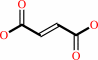 fumarate
fumarate
|
+
|
quinol
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FAD; Iron-sulfur
|
 |
 |
 |
 |
 |
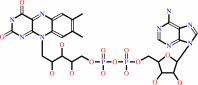 FAD
FAD
|
 Iron-sulfur
Iron-sulfur
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
277:16124-16130
(2002)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystallographic studies of the Escherichia coli quinol-fumarate reductase with inhibitors bound to the quinol-binding site.
|
|
T.M.Iverson,
C.Luna-Chavez,
L.R.Croal,
G.Cecchini,
D.C.Rees.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The quinol-fumarate reductase (QFR) respiratory complex of Escherichia coli is a
four-subunit integral-membrane complex that catalyzes the final step of
anaerobic respiration when fumarate is the terminal electron acceptor. The
membrane-soluble redox-active molecule menaquinol (MQH(2)) transfers electrons
to QFR by binding directly to the membrane-spanning region. The crystal
structure of QFR contains two quinone species, presumably MQH(2), bound to the
transmembrane-spanning region. The binding sites for the two quinone molecules
are termed Q(P) and Q(D), indicating their positions proximal (Q(P)) or distal
(Q(D)) to the site of fumarate reduction in the hydrophilic flavoprotein and
iron-sulfur protein subunits. It has not been established whether both of these
sites are mechanistically significant. Co-crystallization studies of the E. coli
QFR with the known quinol-binding site inhibitors
4,6-dinitrophenol establish that both inhibitors block the binding of MQH(2) at
the Q(P) site. In the structures with the inhibitor bound at Q(P), no density is
observed at Q(D), which suggests that the occupancy of this site can vary and
argues against a structurally obligatory role for quinol binding to Q(D). A
comparison of the Q(P) site of the E. coli enzyme with quinone-binding sites in
other respiratory enzymes shows that an acidic residue is structurally
conserved. This acidic residue, Glu-C29, in the E. coli enzyme may act as a
proton shuttle from the quinol during enzyme turnover.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Fig. 1. Polypeptide -fold and electron transfer distances
in QFR. A, ribbon diagram views of the E. coli QFR separated by
a 90° rotation about a vertical axis. The flavoprotein is
shown in blue, the iron protein is in red, and the transmembrane
anchors are in dark green (FrdC) and purple (FrdD). The
approximate boundary of the membrane is indicated with a black
line. B, inter-cofactor distances of the E. coli enzyme. The
known cofactors are superimposed onto an outline of the enzyme.
C, inter-cofactor distances in the W. succinogenes enzyme. The
b-type hemes associated with the membrane anchor reduce the
electron transfer distance between a predicted distal
quinol-binding site (data not shown). Figs. 1, 3, and 4 were
made using Molscript (56), Bobscript (57), and Raster3D (58).
|
 |
Figure 2.
Fig. 2. Chemical structures for oxidized menaquinone-8
(MQ-8) (A), reduced menaquinol-8 (B), HQNO (C), and DNP-19 (D).
MQ-8 is the primary menaquinone found in E. coli membranes, but
smaller proportions of MQ-6, MQ-7, and MQ-9 are additionally
present in the organism (59).
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2002,
277,
16124-16130)
copyright 2002.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
Z.Itzhaki,
E.Akiva,
and
H.Margalit
(2010).
Preferential use of protein domain pairs as interaction mediators: order and transitivity.
|
| |
Bioinformatics,
26,
2564-2570.
|
 |
|
|
|
|
 |
H.D.Juhnke,
H.Hiltscher,
H.R.Nasiri,
H.Schwalbe,
and
C.R.Lancaster
(2009).
Production, characterization and determination of the real catalytic properties of the putative 'succinate dehydrogenase' from Wolinella succinogenes.
|
| |
Mol Microbiol,
71,
1088-1101.
|
 |
|
|
|
|
 |
Y.L.Chiang,
Y.C.Hsieh,
J.Y.Fang,
E.H.Liu,
Y.C.Huang,
P.Chuankhayan,
J.Jeyakanthan,
M.Y.Liu,
S.I.Chan,
and
C.J.Chen
(2009).
Crystal structure of Adenylylsulfate reductase from Desulfovibrio gigas suggests a potential self-regulation mechanism involving the C terminus of the beta-subunit.
|
| |
J Bacteriol,
191,
7597-7608.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.Jantama,
M.J.Haupt,
S.A.Svoronos,
X.Zhang,
J.C.Moore,
K.T.Shanmugam,
and
L.O.Ingram
(2008).
Combining metabolic engineering and metabolic evolution to develop nonrecombinant strains of Escherichia coli C that produce succinate and malate.
|
| |
Biotechnol Bioeng,
99,
1140-1153.
|
 |
|
|
|
|
 |
T.M.Tomasiak,
E.Maklashina,
G.Cecchini,
and
T.M.Iverson
(2008).
A threonine on the active site loop controls transition state formation in Escherichia coli respiratory complex II.
|
| |
J Biol Chem,
283,
15460-15468.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
Q.M.Tran,
R.A.Rothery,
E.Maklashina,
G.Cecchini,
and
J.H.Weiner
(2007).
Escherichia coli succinate dehydrogenase variant lacking the heme b.
|
| |
Proc Natl Acad Sci U S A,
104,
18007-18012.
|
 |
|
|
|
|
 |
X.Liang,
D.J.Campopiano,
and
P.J.Sadler
(2007).
Metals in membranes.
|
| |
Chem Soc Rev,
36,
968-992.
|
 |
|
|
|
|
 |
A.Oberai,
Y.Ihm,
S.Kim,
and
J.U.Bowie
(2006).
A limited universe of membrane protein families and folds.
|
| |
Protein Sci,
15,
1723-1734.
|
 |
|
|
|
|
 |
L.S.Huang,
G.Sun,
D.Cobessi,
A.C.Wang,
J.T.Shen,
E.Y.Tung,
V.E.Anderson,
and
E.A.Berry
(2006).
3-nitropropionic acid is a suicide inhibitor of mitochondrial respiration that, upon oxidation by complex II, forms a covalent adduct with a catalytic base arginine in the active site of the enzyme.
|
| |
J Biol Chem,
281,
5965-5972.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.L.Rodrigues,
T.F.Oliveira,
I.A.Pereira,
and
M.Archer
(2006).
X-ray structure of the membrane-bound cytochrome c quinol dehydrogenase NrfH reveals novel haem coordination.
|
| |
EMBO J,
25,
5951-5960.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.S.Krishna,
R.I.Sadreyev,
and
N.V.Grishin
(2006).
A tale of two ferredoxins: sequence similarity and structural differences.
|
| |
BMC Struct Biol,
6,
8.
|
 |
|
|
|
|
 |
A.Sevilla,
J.W.Schmid,
K.Mauch,
J.L.Iborra,
M.Reuss,
and
M.Cánovas
(2005).
Model of central and trimethylammonium metabolism for optimizing L-carnitine production by E. coli.
|
| |
Metab Eng,
7,
401-425.
|
 |
|
|
|
|
 |
L.S.Huang,
T.M.Borders,
J.T.Shen,
C.J.Wang,
and
E.A.Berry
(2005).
Crystallization of mitochondrial respiratory complex II from chicken heart: a membrane-protein complex diffracting to 2.0 A.
|
| |
Acta Crystallogr D Biol Crystallogr,
61,
380-387.
|
 |
|
|
|
|
 |
R.A.Rothery,
A.M.Seime,
A.M.Spiers,
E.Maklashina,
I.Schröder,
R.P.Gunsalus,
G.Cecchini,
and
J.H.Weiner
(2005).
Defining the Q-site of Escherichia coli fumarate reductase by site-directed mutagenesis, fluorescence quench titrations and EPR spectroscopy.
|
| |
FEBS J,
272,
313-326.
|
 |
|
|
|
|
 |
R.Giordani,
and
J.Buc
(2004).
Evidence for two different electron transfer pathways in the same enzyme, nitrate reductase A from Escherichia coli.
|
| |
Eur J Biochem,
271,
2400-2407.
|
 |
|
|
|
|
 |
B.D.Silverman
(2003).
Hydrophobicity of transmembrane proteins: spatially profiling the distribution.
|
| |
Protein Sci,
12,
586-599.
|
 |
|
|
|
|
 |
G.Cecchini
(2003).
Function and structure of complex II of the respiratory chain.
|
| |
Annu Rev Biochem,
72,
77.
|
 |
|
|
|
|
 |
H.Miyadera,
K.Shiomi,
H.Ui,
Y.Yamaguchi,
R.Masuma,
H.Tomoda,
H.Miyoshi,
A.Osanai,
K.Kita,
and
S.Omura
(2003).
Atpenins, potent and specific inhibitors of mitochondrial complex II (succinate-ubiquinone oxidoreductase).
|
| |
Proc Natl Acad Sci U S A,
100,
473-477.
|
 |
|
|
|
|
 |
M.G.Almeida,
S.Macieira,
L.L.Gonçalves,
R.Huber,
C.A.Cunha,
M.J.Romão,
C.Costa,
J.Lampreia,
J.J.Moura,
and
I.Moura
(2003).
The isolation and characterization of cytochrome c nitrite reductase subunits (NrfA and NrfH) from Desulfovibrio desulfuricans ATCC 27774. Re-evaluation of the spectroscopic data and redox properties.
|
| |
Eur J Biochem,
270,
3904-3915.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|

| |

