|
Bronsted base
A molecular entity capable of accepting a hydron from a donor (Bronsted acid).
(via organic amino compound )
Bronsted acid
A molecular entity capable of donating a hydron to an acceptor (Bronsted base).
(via oxoacid )
|
|
|
metabolite
Any intermediate or product resulting from metabolism. The term 'metabolite' subsumes the classes commonly known as primary and secondary metabolites.
|
|
succinyladenosine is a Structural Derivative of
|
adenosine
Mass :
267.24152
Formula :
C10H13N5O4

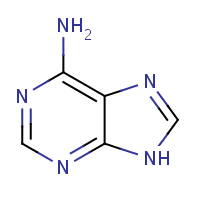
adenine
Mass :
135.12690
Formula :
C5H5N5
carbohydrate
Definition :
Any member of the class of organooxygen compounds that is a polyhydroxy-aldehyde or -ketone or a lactol resulting from their intramolecular condensation (monosaccharides); substances derived from these by reduction of the carbonyl group (alditols), by oxidation of one or more hydroxy groups to afford the corresponding aldehydes, ketones, or carboxylic acids, or by replacement of one or more hydroxy group(s) by a hydrogen atom; and polymeric products arising by intermolecular acetal formation between two or more such molecules (disaccharides, polysaccharides and oligosaccharides). Carbohydrates contain only carbon, hydrogen and oxygen atoms; prior to any oxidation or reduction, most have the empirical formula Cm(H2O)n. Compounds obtained from carbohydrates by substitution, etc., are known as carbohydrate derivatives and may contain other elements. Cyclitols are generally not regarded as carbohydrates.
carbohydrate
Definition :
Any member of the class of organooxygen compounds that is a polyhydroxy-aldehyde or -ketone or a lactol resulting from their intramolecular condensation (monosaccharides); substances derived from these by reduction of the carbonyl group (alditols), by oxidation of one or more hydroxy groups to afford the corresponding aldehydes, ketones, or carboxylic acids, or by replacement of one or more hydroxy group(s) by a hydrogen atom; and polymeric products arising by intermolecular acetal formation between two or more such molecules (disaccharides, polysaccharides and oligosaccharides). Carbohydrates contain only carbon, hydrogen and oxygen atoms; prior to any oxidation or reduction, most have the empirical formula Cm(H2O)n. Compounds obtained from carbohydrates by substitution, etc., are known as carbohydrate derivatives and may contain other elements. Cyclitols are generally not regarded as carbohydrates.
nucleobase
Definition :
That part of DNA or RNA that may be involved in pairing.
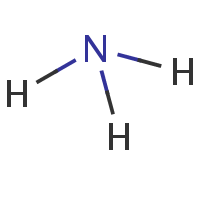
ammonia
Mass :
17.03056
Formula :
H3N
proteinogenic amino acid
Definition :
Any of the 23 alpha-amino acids that are precursors to proteins, and are incorporated into proteins during translation. The group includes the 20 amino acids encoded by the nuclear genes of eukaryotes together with selenocysteine, pyrrolysine, and N-formylmethionine. Apart from glycine, which is non-chiral, all have L configuration.
|
|
succinyladenosine is a Conjugate Acid of
|
|
succinyladenosine anion
Definition :
A dicarboxylic acid anion obtained by deprotonation of at least one of the carboxy groups of succinyladenosine
|
|
|





