 |
PDBsum entry 3vcb

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Viral protein
|
PDB id
|
|

|

|
3vcb
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Viral protein
|
 |
|
Title:
|
 |
C425s mutant of thE C-terminal cytoplasmic domain of non-structural protein 4 from mouse hepatitis virus a59
|
|
Structure:
|
 |
RNA-directed RNA polymerase. Chain: a, b. Fragment: c-terminal cytoplasmic domain. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Murine hepatitis virus. Organism_taxid: 591071. Strain: a59. Gene: murine hepatitis virus strain a59 orf1ab, orf1a. Expressed in: escherichia coli. Expression_system_taxid: 469008.
|
|
Resolution:
|
 |
|
2.40Å
|
R-factor:
|
0.227
|
R-free:
|
0.266
|
|
|
Authors:
|
 |
X.Xu,Z.Lou,Y.Ma,X.Chen,Z.Yang,X.Tong,Q.Zhao,Y.Xu,H.Deng,M.Bartlam, Z.Rao
|
|
Key ref:
|
 |
X.Xu
et al.
(2009).
Crystal structure of the C-terminal cytoplasmic domain of non-structural protein 4 from mouse hepatitis virus A59.
Plos One,
4,
e6217.
PubMed id:
|
 |
|
Date:
|
 |
|
03-Jan-12
|
Release date:
|
11-Jan-12
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P0C6X9
(R1AB_CVMA5) -
Replicase polyprotein 1ab from Murine coronavirus (strain A59)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
7176 a.a.
89 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 2 residue positions (black
crosses)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.2.1.1.56
- mRNA (guanine-N(7))-methyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 5'-end (5'-triphosphoguanosine)-ribonucleoside in mRNA + S-adenosyl-L- methionine = a 5'-end (N(7)-methyl 5'-triphosphoguanosine)-ribonucleoside in mRNA + S-adenosyl-L-homocysteine
|
 |
 |
 |
 |
 |
5'-end (5'-triphosphoguanosine)-ribonucleoside in mRNA
|
+
|
 S-adenosyl-L- methionine
S-adenosyl-L- methionine
|
=
|
5'-end (N(7)-methyl 5'-triphosphoguanosine)-ribonucleoside in mRNA
|
+
|
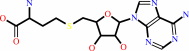 S-adenosyl-L-homocysteine
S-adenosyl-L-homocysteine
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.2.1.1.57
- methyltransferase cap1.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 5'-end (N(7)-methyl 5'-triphosphoguanosine)-ribonucleoside in mRNA + S-adenosyl-L-methionine = a 5'-end (N(7)-methyl 5'-triphosphoguanosine)- (2'-O-methyl-ribonucleoside) in mRNA + S-adenosyl-L-homocysteine + H+
|
 |
 |
 |
 |
 |
5'-end (N(7)-methyl 5'-triphosphoguanosine)-ribonucleoside in mRNA
|
+
|
 S-adenosyl-L-methionine
S-adenosyl-L-methionine
|
=
|
5'-end (N(7)-methyl 5'-triphosphoguanosine)- (2'-O-methyl-ribonucleoside) in mRNA
|
+
|
 S-adenosyl-L-homocysteine
S-adenosyl-L-homocysteine
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 4:
|
 |
E.C.2.7.7.48
- RNA-directed Rna polymerase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
RNA(n) + a ribonucleoside 5'-triphosphate = RNA(n+1) + diphosphate
|
 |
 |
 |
 |
 |
 RNA(n)
RNA(n)
|
+
|
ribonucleoside 5'-triphosphate
|
=
|
RNA(n+1)
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 5:
|
 |
E.C.2.7.7.50
- mRNA guanylyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 5'-end diphospho-ribonucleoside in mRNA + GTP + H+ = a 5'-end (5'-triphosphoguanosine)-ribonucleoside in mRNA + diphosphate
|
 |
 |
 |
 |
 |
5'-end diphospho-ribonucleoside in mRNA
|
+
|
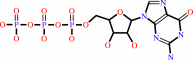 GTP
GTP
|
+
|
H(+)
|
=
|
5'-end (5'-triphosphoguanosine)-ribonucleoside in mRNA
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 6:
|
 |
E.C.3.1.13.-
- ?????
|
|
 |
 |
 |
 |
 |
Enzyme class 7:
|
 |
E.C.3.4.19.12
- ubiquitinyl hydrolase 1.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
Thiol-dependent hydrolysis of ester, thiolester, amide, peptide and isopeptide bonds formed by the C-terminal Gly of ubiquitin (a 76-residue protein attached to proteins as an intracellular targeting signal).
|
 |
 |
 |
 |
 |
Enzyme class 8:
|
 |
E.C.3.4.22.-
- ?????
|
|
 |
 |
 |
 |
 |
Enzyme class 9:
|
 |
E.C.3.6.4.12
- Dna helicase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
ATP + H2O = ADP + phosphate + H+
|
 |
 |
 |
 |
 |
 ATP
ATP
|
+
|
H2O
|
=
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 10:
|
 |
E.C.3.6.4.13
- Rna helicase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
ATP + H2O = ADP + phosphate + H+
|
 |
 |
 |
 |
 |
 ATP
ATP
|
+
|
H2O
|
=
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 11:
|
 |
E.C.4.6.1.-
- ?????
|
|
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Plos One
4:e6217
(2009)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structure of the C-terminal cytoplasmic domain of non-structural protein 4 from mouse hepatitis virus A59.
|
|
X.Xu,
Z.Lou,
Y.Ma,
X.Chen,
Z.Yang,
X.Tong,
Q.Zhao,
Y.Xu,
H.Deng,
M.Bartlam,
Z.Rao.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
BACKGROUND: The replication of coronaviruses takes place on cytoplasmic double
membrane vesicles (DMVs) originating in the endoplasmic reticulum (ER). Three
trans-membrane non-structural proteins, nsp3, nsp4 and nsp6, are understood to
be membrane anchors of the coronavirus replication complex. Nsp4 is localized to
the ER membrane when expressed alone but is recruited into the replication
complex in infected cells. It is revealed to contain four trans-membrane regions
and its N- and C-termini are exposed to the cytosol. METHODOLOGY/PRINCIPAL
FINDINGS: We have determined the crystal structures of the C-terminal
hydrophilic domain of nsp4 (nsp4C) from MHV strain A59 and a C425S site-directed
mutant. The highly conserved 89 amino acid region from T408 to Q496 is shown to
possess a new fold. The wild-type (WT) structure features two monomers linked by
a Cys425-Cys425 disulfide bond in one asymmetric unit. The monomers are arranged
with their N- and C-termini in opposite orientations to form an "open"
conformation. Mutation of Cys425 to Ser did not affect the monomer structure,
although the mutant dimer adopts strikingly different conformations by crystal
packing, with the cross-linked C-termini and parallel N-termini of two monomers
forming a "closed" conformation. The WT nsp4C exists as a dimer in solution and
can dissociate easily into monomers in a reducing environment.
CONCLUSIONS/SIGNIFICANCE: As nsp4C is exposed in the reducing cytosol, the
monomer of nsp4C should be physiological. This structure may serve as a basis
for further functional studies of nsp4.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

