 |
PDBsum entry 1ct9

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Ligase
|
 |
|
Title:
|
 |
Crystal structure of asparagine synthetase b from escherichia coli
|
|
Structure:
|
 |
Asparagine synthetase b. Chain: a, b, c, d. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Escherichia coli. Organism_taxid: 562. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Biol. unit:
|
 |
 Dimer (from
)
Dimer (from
)
|
|
Resolution:
|
 |
|
2.00Å
|
R-factor:
|
0.197
|
R-free:
|
0.297
|
|
|
Authors:
|
 |
T.M.Larsen,S.K.Boehlein,S.M.Schuster,N.G.J.Richards,J.B.Thoden, H.M.Holden,I.Rayment
|
Key ref:

|
 |
T.M.Larsen
et al.
(1999).
Three-dimensional structure of Escherichia coli asparagine synthetase B: a short journey from substrate to product.
Biochemistry,
38,
16146-16157.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
20-Aug-99
|
Release date:
|
15-Dec-99
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P22106
(ASNB_ECOLI) -
Asparagine synthetase B [glutamine-hydrolyzing] from Escherichia coli (strain K12)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
554 a.a.
497 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.3.5.4
- asparagine synthase (glutamine-hydrolyzing).
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
L-aspartate + L-glutamine + ATP + H2O = L-asparagine + L-glutamate + AMP + diphosphate + H+
|
 |
 |
 |
 |
 |
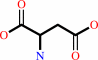 L-aspartate
L-aspartate
|
+
|
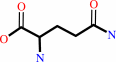 L-glutamine
L-glutamine
|
+
|
 ATP
ATP
|
+
|
H2O
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
L-asparagine
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
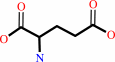 L-glutamate
L-glutamate
|
+
|
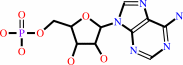 AMP
AMP
|
+
|
 diphosphate
diphosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
38:16146-16157
(1999)
|
|
PubMed id:
|
|
|
|

|
| |
|
Three-dimensional structure of Escherichia coli asparagine synthetase B: a short journey from substrate to product.
|
|
T.M.Larsen,
S.K.Boehlein,
S.M.Schuster,
N.G.Richards,
J.B.Thoden,
H.M.Holden,
I.Rayment.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Asparagine synthetase B catalyzes the assembly of asparagine from aspartate,
Mg(2+)ATP, and glutamine. Here, we describe the three-dimensional structure of
the enzyme from Escherichia colidetermined and refined to 2.0 A resolution.
Protein employed for this study was that of a site-directed mutant protein,
Cys1Ala. Large crystals were grown in the presence of both glutamine and AMP.
Each subunit of the dimeric protein folds into two distinct domains. The
N-terminal region contains two layers of antiparallel beta-sheet with each layer
containing six strands. Wedged between these layers of sheet is the active site
responsible for the hydrolysis of glutamine. Key side chains employed for
positioning the glutamine substrate within the binding pocket include Arg 49,
Asn 74, Glu 76, and Asp 98. The C-terminal domain, responsible for the binding
of both Mg(2+)ATP and aspartate, is dominated by a five-stranded parallel
beta-sheet flanked on either side by alpha-helices. The AMP moiety is anchored
to the protein via hydrogen bonds with O(gamma) of Ser 346 and the backbone
carbonyl and amide groups of Val 272, Leu 232, and Gly 347. As observed for
other amidotransferases, the two active sites are connected by a tunnel lined
primarily with backbone atoms and hydrophobic and nonpolar amino acid residues.
Strikingly, the three-dimensional architecture of the N-terminal domain of
asparagine synthetase B is similar to that observed for glutamine
phosphoribosylpyrophosphate amidotransferase while the molecular motif of the
C-domain is reminiscent to that observed for GMP synthetase.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
B.Sagot,
M.Gaysinski,
M.Mehiri,
J.M.Guigonis,
D.Le Rudulier,
and
G.Alloing
(2010).
Osmotically induced synthesis of the dipeptide N-acetylglutaminylglutamine amide is mediated by a new pathway conserved among bacteria.
|
| |
Proc Natl Acad Sci U S A,
107,
12652-12657.
|
 |
|
|
|
|
 |
L.Lund,
Y.Fan,
Q.Shao,
Y.Q.Gao,
and
F.M.Raushel
(2010).
Carbamate transport in carbamoyl phosphate synthetase: a theoretical and experimental investigation.
|
| |
J Am Chem Soc,
132,
3870-3878.
|
 |
|
|
|
|
 |
M.L.Raber,
S.O.Arnett,
and
C.A.Townsend
(2009).
A conserved tyrosyl-glutamyl catalytic dyad in evolutionarily linked enzymes: carbapenam synthetase and beta-lactam synthetase.
|
| |
Biochemistry,
48,
4959-4971.
|
 |
|
|
|
|
 |
N.LaRonde-LeBlanc,
M.Resto,
and
B.Gerratana
(2009).
Regulation of active site coupling in glutamine-dependent NAD(+) synthetase.
|
| |
Nat Struct Mol Biol,
16,
421-429.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.K.Lim,
J.Ju,
E.Zazopoulos,
H.Jiang,
J.W.Seo,
Y.Chen,
Z.Feng,
S.R.Rajski,
C.M.Farnet,
and
B.Shen
(2009).
iso-Migrastatin, migrastatin, and dorrigocin production in Streptomyces platensis NRRL 18993 is governed by a single biosynthetic machinery featuring an acyltransferase-less type I polyketide synthase.
|
| |
J Biol Chem,
284,
29746-29756.
|
 |
|
|
|
|
 |
T.A.Knappe,
U.Linne,
L.Robbel,
and
M.A.Marahiel
(2009).
Insights into the biosynthesis and stability of the lasso peptide capistruin.
|
| |
Chem Biol,
16,
1290-1298.
|
 |
|
|
|
|
 |
Y.Fan,
L.Lund,
Q.Shao,
Y.Q.Gao,
and
F.M.Raushel
(2009).
A combined theoretical and experimental study of the ammonia tunnel in carbamoyl phosphate synthetase.
|
| |
J Am Chem Soc,
131,
10211-10219.
|
 |
|
|
|
|
 |
K.Severinov,
E.Semenova,
A.Kazakov,
T.Kazakov,
and
M.S.Gelfand
(2007).
Low-molecular-weight post-translationally modified microcins.
|
| |
Mol Microbiol,
65,
1380-1394.
|
 |
|
|
|
|
 |
S.Duquesne,
D.Destoumieux-Garzón,
S.Zirah,
C.Goulard,
J.Peduzzi,
and
S.Rebuffat
(2007).
Two enzymes catalyze the maturation of a lasso peptide in Escherichia coli.
|
| |
Chem Biol,
14,
793-803.
|
 |
|
|
|
|
 |
S.Mouilleron,
and
B.Golinelli-Pimpaneau
(2007).
Conformational changes in ammonia-channeling glutamine amidotransferases.
|
| |
Curr Opin Struct Biol,
17,
653-664.
|
 |
|
|
|
|
 |
A.Nakamura,
M.Yao,
S.Chimnaronk,
N.Sakai,
and
I.Tanaka
(2006).
Ammonia channel couples glutaminase with transamidase reactions in GatCAB.
|
| |
Science,
312,
1954-1958.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
H.Ren,
and
J.Liu
(2006).
AsnB is involved in natural resistance of Mycobacterium smegmatis to multiple drugs.
|
| |
Antimicrob Agents Chemother,
50,
250-255.
|
 |
|
|
|
|
 |
J.A.Gutierrez,
Y.X.Pan,
L.Koroniak,
J.Hiratake,
M.S.Kilberg,
and
N.G.Richards
(2006).
An inhibitor of human asparagine synthetase suppresses proliferation of an L-asparaginase-resistant leukemia cell line.
|
| |
Chem Biol,
13,
1339-1347.
|
 |
|
|
|
|
 |
J.D.Mougous,
D.H.Lee,
S.C.Hubbard,
M.W.Schelle,
D.J.Vocadlo,
J.M.Berger,
and
C.R.Bertozzi
(2006).
Molecular basis for G protein control of the prokaryotic ATP sulfurylase.
|
| |
Mol Cell,
21,
109-122.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
N.G.Richards,
and
M.S.Kilberg
(2006).
Asparagine synthetase chemotherapy.
|
| |
Annu Rev Biochem,
75,
629-654.
|
 |
|
|
|
|
 |
R.A.Cañas,
F.de la Torre,
F.M.Cánovas,
and
F.R.Cantón
(2006).
High levels of asparagine synthetase in hypocotyls of pine seedlings suggest a role of the enzyme in re-allocation of seed-stored nitrogen.
|
| |
Planta,
224,
83-95.
|
 |
|
|
|
|
 |
W.Zhang,
B.D.Ames,
S.C.Tsai,
and
Y.Tang
(2006).
Engineered biosynthesis of a novel amidated polyketide, using the malonamyl-specific initiation module from the oxytetracycline polyketide synthase.
|
| |
Appl Environ Microbiol,
72,
2573-2580.
|
 |
|
|
|
|
 |
F.Levitin,
O.Stern,
M.Weiss,
C.Gil-Henn,
R.Ziv,
Z.Prokocimer,
N.I.Smorodinsky,
D.B.Rubinstein,
and
D.H.Wreschner
(2005).
The MUC1 SEA module is a self-cleaving domain.
|
| |
J Biol Chem,
280,
33374-33386.
|
 |
|
|
|
|
 |
N.J.Kershaw,
M.E.Caines,
M.C.Sleeman,
and
C.J.Schofield
(2005).
The enzymology of clavam and carbapenem biosynthesis.
|
| |
Chem Commun (Camb),
(),
4251-4263.
|
 |
|
|
|
|
 |
Y.Mitani,
X.Meng,
Y.Kamagata,
and
T.Tamura
(2005).
Characterization of LtsA from Rhodococcus erythropolis, an enzyme with glutamine amidotransferase activity.
|
| |
J Bacteriol,
187,
2582-2591.
|
 |
|
|
|
|
 |
E.L.Hendrickson,
R.Kaul,
Y.Zhou,
D.Bovee,
P.Chapman,
J.Chung,
E.Conway de Macario,
J.A.Dodsworth,
W.Gillett,
D.E.Graham,
M.Hackett,
A.K.Haydock,
A.Kang,
M.L.Land,
R.Levy,
T.J.Lie,
T.A.Major,
B.C.Moore,
I.Porat,
A.Palmeiri,
G.Rouse,
C.Saenphimmachak,
D.Söll,
S.Van Dien,
T.Wang,
W.B.Whitman,
Q.Xia,
Y.Zhang,
F.W.Larimer,
M.V.Olson,
and
J.A.Leigh
(2004).
Complete genome sequence of the genetically tractable hydrogenotrophic methanogen Methanococcus maripaludis.
|
| |
J Bacteriol,
186,
6956-6969.
|
 |
|
|
|
|
 |
F.A.Lunn,
and
S.L.Bearne
(2004).
Alternative substrates for wild-type and L109A E. coli CTP synthases: kinetic evidence for a constricted ammonia tunnel.
|
| |
Eur J Biochem,
271,
4204-4212.
|
 |
|
|
|
|
 |
H.H.Lin,
G.W.Chang,
J.Q.Davies,
M.Stacey,
J.Harris,
and
S.Gordon
(2004).
Autocatalytic cleavage of the EMR2 receptor occurs at a conserved G protein-coupled receptor proteolytic site motif.
|
| |
J Biol Chem,
279,
31823-31832.
|
 |
|
|
|
|
 |
J.D.Lawson,
E.Pate,
I.Rayment,
and
R.G.Yount
(2004).
Molecular dynamics analysis of structural factors influencing back door pi release in myosin.
|
| |
Biophys J,
86,
3794-3803.
|
 |
|
|
|
|
 |
V.M.Coiro,
A.Di Nola,
M.A.Vanoni,
M.Aschi,
A.Coda,
B.Curti,
and
D.Roccatano
(2004).
Molecular dynamics simulation of the interaction between the complex iron-sulfur flavoprotein glutamate synthase and its substrates.
|
| |
Protein Sci,
13,
2979-2991.
|
 |
|
|
|
|
 |
B.A.Manjasetty,
J.Powlowski,
and
A.Vrielink
(2003).
Crystal structure of a bifunctional aldolase-dehydrogenase: sequestering a reactive and volatile intermediate.
|
| |
Proc Natl Acad Sci U S A,
100,
6992-6997.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
D.E.Pilloff,
and
T.S.Leyh
(2003).
Allosteric and catalytic functions of the PPi-binding motif in the ATP sulfurylase-GTPase system.
|
| |
J Biol Chem,
278,
50435-50441.
|
 |
|
|
|
|
 |
M.Goto,
R.Omi,
I.Miyahara,
M.Sugahara,
and
K.Hirotsu
(2003).
Structures of argininosuccinate synthetase in enzyme-ATP substrates and enzyme-AMP product forms: stereochemistry of the catalytic reaction.
|
| |
J Biol Chem,
278,
22964-22971.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.T.Miller,
B.Gerratana,
A.Stapon,
C.A.Townsend,
and
A.C.Rosenzweig
(2003).
Crystal structure of carbapenam synthetase (CarA).
|
| |
J Biol Chem,
278,
40996-41002.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.Amaro,
E.Tajkhorshid,
and
Z.Luthey-Schulten
(2003).
Developing an energy landscape for the novel function of a (beta/alpha)8 barrel: ammonia conduction through HisF.
|
| |
Proc Natl Acad Sci U S A,
100,
7599-7604.
|
 |
|
|
|
|
 |
A.Douangamath,
M.Walker,
S.Beismann-Driemeyer,
M.C.Vega-Fernandez,
R.Sterner,
and
M.Wilmanns
(2002).
Structural evidence for ammonia tunneling across the (beta alpha)(8) barrel of the imidazole glycerol phosphate synthase bienzyme complex.
|
| |
Structure,
10,
185-193.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Magalon,
C.Frixon,
J.Pommier,
G.Giordano,
and
F.Blasco
(2002).
In vivo interactions between gene products involved in the final stages of molybdenum cofactor biosynthesis in Escherichia coli.
|
| |
J Biol Chem,
277,
48199-48204.
|
 |
|
|
|
|
 |
B.Min,
J.T.Pelaschier,
D.E.Graham,
D.Tumbula-Hansen,
and
D.Söll
(2002).
Transfer RNA-dependent amino acid biosynthesis: an essential route to asparagine formation.
|
| |
Proc Natl Acad Sci U S A,
99,
2678-2683.
|
 |
|
|
|
|
 |
C.A.Townsend
(2002).
New reactions in clavulanic acid biosynthesis.
|
| |
Curr Opin Chem Biol,
6,
583-589.
|
 |
|
|
|
|
 |
C.T.Lemke,
and
P.L.Howell
(2002).
Substrate induced conformational changes in argininosuccinate synthetase.
|
| |
J Biol Chem,
277,
13074-13081.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.B.Thoden,
X.Huang,
F.M.Raushel,
and
H.M.Holden
(2002).
Carbamoyl-phosphate synthetase. Creation of an escape route for ammonia.
|
| |
J Biol Chem,
277,
39722-39727.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.O.Broschat,
C.Gorka,
J.D.Page,
C.L.Martin-Berger,
M.S.Davies,
H.C.Huang Hc,
E.A.Gulve,
W.J.Salsgiver,
and
T.P.Kasten
(2002).
Kinetic characterization of human glutamine-fructose-6-phosphate amidotransferase I: potent feedback inhibition by glucosamine 6-phosphate.
|
| |
J Biol Chem,
277,
14764-14770.
|
 |
|
|
|
|
 |
M.Goto,
Y.Nakajima,
and
K.Hirotsu
(2002).
Crystal structure of argininosuccinate synthetase from Thermus thermophilus HB8. Structural basis for the catalytic action.
|
| |
J Biol Chem,
277,
15890-15896.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.T.Miller,
B.O.Bachmann,
C.A.Townsend,
and
A.C.Rosenzweig
(2002).
The catalytic cycle of beta -lactam synthetase observed by x-ray crystallographic snapshots.
|
| |
Proc Natl Acad Sci U S A,
99,
14752-14757.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.H.van den Heuvel,
D.Ferrari,
R.T.Bossi,
S.Ravasio,
B.Curti,
M.A.Vanoni,
F.J.Florencio,
and
A.Mattevi
(2002).
Structural studies on the synchronization of catalytic centers in glutamate synthase.
|
| |
J Biol Chem,
277,
24579-24583.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
X.Huang,
H.M.Holden,
and
F.M.Raushel
(2001).
Channeling of substrates and intermediates in enzyme-catalyzed reactions.
|
| |
Annu Rev Biochem,
70,
149-180.
|
 |
|
|
|
|
 |
A.F.Kisselev,
Z.Songyang,
and
A.L.Goldberg
(2000).
Why does threonine, and not serine, function as the active site nucleophile in proteasomes?
|
| |
J Biol Chem,
275,
14831-14837.
|
 |
|
|
|
|
 |
A.K.Bera,
S.Chen,
J.L.Smith,
and
H.Zalkin
(2000).
Temperature-dependent function of the glutamine phosphoribosylpyrophosphate amidotransferase ammonia channel and coupling with glycinamide ribonucleotide synthetase in a hyperthermophile.
|
| |
J Bacteriol,
182,
3734-3739.
|
 |
|
|
|
|
 |
B.O.Bachmann,
and
C.A.Townsend
(2000).
Kinetic mechanism of the beta-lactam synthetase of Streptomyces clavuligerus.
|
| |
Biochemistry,
39,
11187-11193.
|
 |
|
|
|
|
 |
C.Binda,
R.T.Bossi,
S.Wakatsuki,
S.Arzt,
A.Coda,
B.Curti,
M.A.Vanoni,
and
A.Mattevi
(2000).
Cross-talk and ammonia channeling between active centers in the unexpected domain arrangement of glutamate synthase.
|
| |
Structure,
8,
1299-1308.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

