 |
PDBsum entry 1kh3

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.3.4.5
- argininosuccinate synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Urea Cycle and Arginine Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
L-citrulline + L-aspartate + ATP = 2-(N(omega)-L-arginino)succinate + AMP + diphosphate + H+
|
 |
 |
 |
 |
 |
 L-citrulline
L-citrulline
|
+
|
L-aspartate
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
ATP
Bound ligand (Het Group name = )
matches with 84.62% similarity
|
=
|
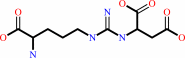 2-(N(omega)-L-arginino)succinate
2-(N(omega)-L-arginino)succinate
|
+
|
AMP
Bound ligand (Het Group name = )
matches with 74.19% similarity
|
+
|
 diphosphate
diphosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
278:22964-22971
(2003)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structures of argininosuccinate synthetase in enzyme-ATP substrates and enzyme-AMP product forms: stereochemistry of the catalytic reaction.
|
|
M.Goto,
R.Omi,
I.Miyahara,
M.Sugahara,
K.Hirotsu.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Argininosuccinate synthetase reversibly catalyzes the ATP-dependent condensation
of a citrulline with an aspartate to give argininosuccinate. The structures of
the enzyme from Thermus thermophilus HB8 complexed with intact ATP and
substrates (citrulline and aspartate) and with AMP and product
(argininosuccinate) have been determined at 2.1- and 2.0-A resolution,
respectively. The enzyme does not show the ATP-induced domain rotation observed
in the enzyme from Escherichia coli. In the enzyme-substrate complex, the
reaction sites of ATP and the bound substrates are adjacent and are sufficiently
close for the reaction to proceed without the large conformational change at the
domain level. The mobility of the triphosphate group in ATP and the side chain
of citrulline play an important role in the catalytic action. The protonated
amino group of the bound aspartate interacts with the alpha-phosphate of ATP and
the ureido group of citrulline, thus stimulating the adenylation of citrulline.
The enzyme-product complex explains how the citrullyl-AMP intermediate is bound
to the active site. The stereochemistry of the catalysis of the enzyme is
clarified on the basis of the structures of tAsS (argininosuccinate synthetase
from T. thermophilus HB8) complexes.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 5.
FIG. 5. The superposition of the substrate analogue
(arginine) in
tAsS·AMP-PNP·arginine·succinate onto the
substrate (citrulline) in
tAsS·ATP·citrulline·aspartate. The
arrangement of arginine (heavily shaded), ATP, and substrates
(citrulline and aspartate) is displayed. Arginine might imitate
the approach of citrulline to the  -phosphate of ATP.
Important short contacts are shown by dotted lines. -phosphate of ATP.
Important short contacts are shown by dotted lines.
|
 |
Figure 6.
FIG. 6. Proposed stereochemistry along the catalytic
process based on x-ray structures of tAsS complexes. Putative
hydrogen bonds are shown by dotted lines. A, tAsS complex with
U-shaped ATP and citrulline. B, tAsS complex with U-shaped ATP,
citrulline, and aspartate. Aspartate is adjacent to the  -phosphate of ATP and
the ureido group of citrulline. C and D, tAsS complex with
S-shaped ATP, citrulline, and aspartate. ATP changes its
triphosphate conformation. D, citrulline changes its side-chain
conformation to approach the -phosphate of ATP and
the ureido group of citrulline. C and D, tAsS complex with
S-shaped ATP, citrulline, and aspartate. ATP changes its
triphosphate conformation. D, citrulline changes its side-chain
conformation to approach the  -phosphate of ATP (D).
E, tAsS complex with citrullyl-AMP intermediate. F, tAsS complex
with AMP and the product argininosuccinate. -phosphate of ATP (D).
E, tAsS complex with citrullyl-AMP intermediate. F, tAsS complex
with AMP and the product argininosuccinate.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2003,
278,
22964-22971)
copyright 2003.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
T.Karlberg,
R.Collins,
S.van den Berg,
A.Flores,
M.Hammarström,
M.Högbom,
L.Holmberg Schiavone,
and
J.Uppenberg
(2008).
Structure of human argininosuccinate synthetase.
|
| |
Acta Crystallogr D Biol Crystallogr,
64,
279-286.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.Kuratani,
Y.Yoshikawa,
Y.Bessho,
K.Higashijima,
T.Ishii,
R.Shibata,
S.Takahashi,
K.Yutani,
and
S.Yokoyama
(2007).
Structural basis of the initial binding of tRNA(Ile) lysidine synthetase TilS with ATP and L-lysine.
|
| |
Structure,
15,
1642-1653.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
N.G.Richards,
and
M.S.Kilberg
(2006).
Asparagine synthetase chemotherapy.
|
| |
Annu Rev Biochem,
75,
629-654.
|
 |
|
|
|
|
 |
E.Curis,
I.Nicolis,
C.Moinard,
S.Osowska,
N.Zerrouk,
S.Bénazeth,
and
L.Cynober
(2005).
Almost all about citrulline in mammals.
|
| |
Amino Acids,
29,
177-205.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

