 |
PDBsum entry 2tsc

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Transferase (methyltransferase)
|
PDB id
|
|

|

|
2tsc
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.1.1.45
- thymidylate synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Folate Coenzymes
|
 |
 |
 |
 |
 |
Reaction:
|
 |
dUMP + (6R)-5,10-methylene-5,6,7,8-tetrahydrofolate = 7,8-dihydrofolate + dTMP
|
 |
 |
 |
 |
 |
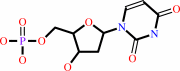 dUMP
dUMP
|
+
|
(6R)-5,10-methylene-5,6,7,8-tetrahydrofolate
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
7,8-dihydrofolate
Bound ligand (Het Group name = )
matches with 48.89% similarity
|
+
|
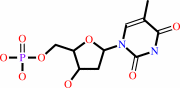 dTMP
dTMP
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
29:6964-6977
(1990)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure, multiple site binding, and segmental accommodation in thymidylate synthase on binding dUMP and an anti-folate.
|
|
W.R.Montfort,
K.M.Perry,
E.B.Fauman,
J.S.Finer-Moore,
G.F.Maley,
L.Hardy,
F.Maley,
R.M.Stroud.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The structure of Escherichia coli thymidylate synthase (TS) complexed with the
substrate dUMP and an analogue of the cofactor methylenetetrahydrofolate was
solved by multiple isomorphous replacement and refined at 1.97-A resolution to a
residual of 18% for all data (16% for data greater than 2 sigma) for a highly
constrained structure. All residues in the structure are clearly resolved and
give a very high confidence in total correctness of the structure. The ternary
complex directly suggests how methylation of dUMP takes place. C-6 of dUMP is
covalently bound to gamma S of Cys-198(146) during catalysis, and the reactants
are surrounded by specific hydrogen bonds and hydrophobic interactions from
conserved residues. Comparison with the independently solved structure of
unliganded TS reveals a large conformation change in the enzyme, which closes
down to sequester the reactants and several highly ordered water molecules
within a cavernous active center, away from bulk solvent. A second binding site
for the quinazoline ring of the cofactor analogue was discovered by withholding
addition of reducing agent during crystal storage. The chemical change in the
protein is slight, and from difference density maps modification of sulfhydryls
is not directly responsible for blockade of the primary site. The site, only
partially overlapping with the primary site, is also surrounded by conserved
residues and thus may play a functional role. The ligand-induced conformational
change is not a domain shift but involves the segmental accommodation of several
helices, beta-strands, and loops that move as units against the beta-sheet
interface between monomers.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
B.Luo,
J.Repalli,
A.M.Yousef,
S.R.Johnson,
L.Lebioda,
and
S.H.Berger
(2011).
Human thymidylate synthase with loop 181-197 stabilized in an inactive conformation: ligand interactions, phosphorylation, and inhibition profiles.
|
| |
Protein Sci,
20,
87-94.
|
 |
|
|
|
|
 |
B.Luo,
S.R.Johnson,
L.Lebioda,
and
S.H.Berger
(2011).
Evolution of metamorphism in thymidylate synthases within the primate lineages.
|
| |
J Mol Evol,
72,
306-314.
|
 |
|
|
|
|
 |
J.D.Chodera,
D.L.Mobley,
M.R.Shirts,
R.W.Dixon,
K.Branson,
and
V.S.Pande
(2011).
Alchemical free energy methods for drug discovery: progress and challenges.
|
| |
Curr Opin Struct Biol,
21,
150-160.
|
 |
|
|
|
|
 |
M.Chanama,
S.Chanama,
P.J.Shaw,
P.Chitnumsub,
U.Leartsakulpanich,
and
Y.Yuthavong
(2011).
Formation of catalytically active cross-species heterodimers of thymidylate synthase from Plasmodium falciparum and Plasmodium vivax.
|
| |
Mol Biol Rep,
38,
1029-1037.
|
 |
|
|
|
|
 |
F.L.Mitchell,
S.M.Miles,
J.Neres,
E.V.Bichenkova,
and
R.A.Bryce
(2010).
Tryptophan as a molecular shovel in the glycosyl transfer activity of Trypanosoma cruzi trans-sialidase.
|
| |
Biophys J,
98,
L38-L40.
|
 |
|
|
|
|
 |
N.Kanaan,
M.Roca,
I.Tuñón,
S.Martí,
and
V.Moliner
(2010).
Theoretical study of the temperature dependence of dynamic effects in thymidylate synthase.
|
| |
Phys Chem Chem Phys,
12,
11657-11664.
|
 |
|
|
|
|
 |
A.Alian,
A.DeGiovanni,
S.L.Griner,
J.S.Finer-Moore,
and
R.M.Stroud
(2009).
Crystal structure of an RluF-RNA complex: a base-pair rearrangement is the key to selectivity of RluF for U2604 of the ribosome.
|
| |
J Mol Biol,
388,
785-800.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
D.L.Mobley,
and
K.A.Dill
(2009).
Binding of small-molecule ligands to proteins: "what you see" is not always "what you get".
|
| |
Structure,
17,
489-498.
|
 |
|
|
|
|
 |
K.V.Kudryavtsev,
M.L.Bentley,
and
D.G.McCafferty
(2009).
Probing of the cis-5-phenyl proline scaffold as a platform for the synthesis of mechanism-based inhibitors of the Staphylococcus aureus sortase SrtA isoform.
|
| |
Bioorg Med Chem,
17,
2886-2893.
|
 |
|
|
|
|
 |
M.N.Lee,
D.Takawira,
A.P.Nikolova,
D.P.Ballou,
V.C.Furtado,
N.L.Phung,
B.R.Still,
M.K.Thorstad,
J.J.Tanner,
and
E.E.Trimmer
(2009).
Functional role for the conformationally mobile phenylalanine 223 in the reaction of methylenetetrahydrofolate reductase from Escherichia coli.
|
| |
Biochemistry,
48,
7673-7685.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.A.Arvizu-Flores,
R.Sugich-Miranda,
R.Arreola,
K.D.Garcia-Orozco,
E.F.Velazquez-Contreras,
W.R.Montfort,
F.Maley,
and
R.R.Sotelo-Mundo
(2008).
Role of an invariant lysine residue in folate binding on Escherichia coli thymidylate synthase: calorimetric and crystallographic analysis of the K48Q mutant.
|
| |
Int J Biochem Cell Biol,
40,
2206-2217.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Alian,
T.T.Lee,
S.L.Griner,
R.M.Stroud,
and
J.Finer-Moore
(2008).
Structure of a TrmA-RNA complex: A consensus RNA fold contributes to substrate selectivity and catalysis in m5U methyltransferases.
|
| |
Proc Natl Acad Sci U S A,
105,
6876-6881.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
W.E.Martucci,
M.A.Vargo,
and
K.S.Anderson
(2008).
Explaining an unusually fast parasitic enzyme: folate tail-binding residues dictate substrate positioning and catalysis in Cryptosporidium hominis thymidylate synthase.
|
| |
Biochemistry,
47,
8902-8911.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.L.Lovelace,
L.M.Gibson,
and
L.Lebioda
(2007).
Cooperative inhibition of human thymidylate synthase by mixtures of active site binding and allosteric inhibitors.
|
| |
Biochemistry,
46,
2823-2830.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.L.Saxl,
G.F.Maley,
C.R.Hauer,
R.Maccoll,
L.Changchien,
and
F.Maley
(2007).
Significance of mutations on the structural perturbation of thymidylate synthase: implications for their involvement in subunit exchange.
|
| |
Protein Sci,
16,
1439-1448.
|
 |
|
|
|
|
 |
R.R.Sotelo-Mundo,
L.Changchien,
F.Maley,
and
W.R.Montfort
(2006).
Crystal structures of thymidylate synthase mutant R166Q: structural basis for the nearly complete loss of catalytic activity.
|
| |
J Biochem Mol Toxicol,
20,
88-92.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.A.Roberts,
D.C.Hyatt,
J.E.Honts,
L.Changchien,
G.F.Maley,
F.Maley,
and
W.R.Montfort
(2006).
Structure of the Y94F mutant of Escherichia coli thymidylate synthase.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
62,
840-843.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.S.Finer-Moore,
A.C.Anderson,
R.H.O'Neil,
M.P.Costi,
S.Ferrari,
J.Krucinski,
and
R.M.Stroud
(2005).
The structure of Cryptococcus neoformans thymidylate synthase suggests strategies for using target dynamics for species-specific inhibition.
|
| |
Acta Crystallogr D Biol Crystallogr,
61,
1320-1334.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.H.Lilien,
B.W.Stevens,
A.C.Anderson,
and
B.R.Donald
(2005).
A novel ensemble-based scoring and search algorithm for protein redesign and its application to modify the substrate specificity of the gramicidin synthetase a phenylalanine adenylation enzyme.
|
| |
J Comput Biol,
12,
740-761.
|
 |
|
|
|
|
 |
C.E.Atreya,
E.F.Johnson,
J.Williamson,
S.Y.Chang,
P.H.Liang,
and
K.S.Anderson
(2003).
Probing electrostatic channeling in protozoal bifunctional thymidylate synthase-dihydrofolate reductase using site-directed mutagenesis.
|
| |
J Biol Chem,
278,
28901-28911.
|
 |
|
|
|
|
 |
R.H.O'Neil,
R.H.Lilien,
B.R.Donald,
R.M.Stroud,
and
A.C.Anderson
(2003).
Phylogenetic classification of protozoa based on the structure of the linker domain in the bifunctional enzyme, dihydrofolate reductase-thymidylate synthase.
|
| |
J Biol Chem,
278,
52980-52987.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.H.O'Neil,
R.H.Lilien,
B.R.Donald,
R.M.Stroud,
and
A.C.Anderson
(2003).
The crystal structure of dihydrofolate reductase-thymidylate synthase from Cryptosporidium hominis reveals a novel architecture for the bifunctional enzyme.
|
| |
J Eukaryot Microbiol,
50,
555-556.
|
 |
|
|
|
|
 |
S.Ferrari,
P.M.Costi,
and
R.C.Wade
(2003).
Inhibitor specificity via protein dynamics: insights from the design of antibacterial agents targeted against thymidylate synthase.
|
| |
Chem Biol,
10,
1183-1193.
|
 |
|
|
|
|
 |
X.Lin,
J.Liu,
F.Maley,
and
E.Chu
(2003).
Role of cysteine amino acid residues on the RNA binding activity of human thymidylate synthase.
|
| |
Nucleic Acids Res,
31,
4882-4887.
|
 |
|
|
|
|
 |
E.F.Johnson,
W.Hinz,
C.E.Atreya,
F.Maley,
and
K.S.Anderson
(2002).
Mechanistic characterization of Toxoplasma gondii thymidylate synthase (TS-DHFR)-dihydrofolate reductase. Evidence for a TS intermediate and TS half-sites reactivity.
|
| |
J Biol Chem,
277,
43126-43136.
|
 |
|
|
|
|
 |
T.Lazaridis,
A.Masunov,
and
F.Gandolfo
(2002).
Contributions to the binding free energy of ligands to avidin and streptavidin.
|
| |
Proteins,
47,
194-208.
|
 |
|
|
|
|
 |
A.C.Anderson,
R.H.O'Neil,
T.S.Surti,
and
R.M.Stroud
(2001).
Approaches to solving the rigid receptor problem by identifying a minimal set of flexible residues during ligand docking.
|
| |
Chem Biol,
8,
445-457.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
G.Sengle,
A.Eisenführ,
P.S.Arora,
J.S.Nowick,
and
M.Famulok
(2001).
Novel RNA catalysts for the Michael reaction.
|
| |
Chem Biol,
8,
459-473.
|
 |
|
|
|
|
 |
K.Aso,
Y.Imai,
K.Yukishige,
K.Ootsu,
and
H.Akimoto
(2001).
Pyrrolo[2,3-d]pyrimidine thymidylate synthase inhibitors: design and synthesis of one-carbon bridge derivatives.
|
| |
Chem Pharm Bull (Tokyo),
49,
1280-1287.
|
 |
|
|
|
|
 |
R.Almog,
C.A.Waddling,
F.Maley,
G.F.Maley,
and
P.Van Roey
(2001).
Crystal structure of a deletion mutant of human thymidylate synthase Delta (7-29) and its ternary complex with Tomudex and dUMP.
|
| |
Protein Sci,
10,
988-996.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.A.Fritz,
D.Tondi,
J.S.Finer-Moore,
M.P.Costi,
and
R.M.Stroud
(2001).
Predicting and harnessing protein flexibility in the design of species-specific inhibitors of thymidylate synthase.
|
| |
Chem Biol,
8,
981-995.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
D.A.Erlanson,
A.C.Braisted,
D.R.Raphael,
M.Randal,
R.M.Stroud,
E.M.Gordon,
and
J.A.Wells
(2000).
Site-directed ligand discovery.
|
| |
Proc Natl Acad Sci U S A,
97,
9367-9372.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.Phan,
E.Mahdavian,
M.C.Nivens,
W.Minor,
S.Berger,
H.T.Spencer,
R.B.Dunlap,
and
L.Lebioda
(2000).
Catalytic cysteine of thymidylate synthase is activated upon substrate binding.
|
| |
Biochemistry,
39,
6969-6978.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.J.Morse,
S.Kawase,
D.V.Santi,
J.Finer-Moore,
and
R.M.Stroud
(2000).
Energetic contributions of four arginines to phosphate-binding in thymidylate synthase are more than additive and depend on optimization of "effective charge balance".
|
| |
Biochemistry,
39,
1011-1020.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
B.W.Shen,
D.H.Dyer,
J.Y.Huang,
L.D'Ari,
J.Rabinowitz,
and
B.L.Stoddard
(1999).
The crystal structure of a bacterial, bifunctional 5,10 methylene-tetrahydrofolate dehydrogenase/cyclohydrolase.
|
| |
Protein Sci,
8,
1342-1349.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
D.J.Steadman,
H.T.Spencer,
R.B.Dunlap,
and
S.H.Berger
(1999).
Substitution at residue 214 of human thymidylate synthase alters nucleotide binding and isomerization of ligand-protein complexes.
|
| |
Biochemistry,
38,
5582-5587.
|
 |
|
|
|
|
 |
H.K.Song,
S.H.Sohn,
and
S.W.Suh
(1999).
Crystal structure of deoxycytidylate hydroxymethylase from bacteriophage T4, a component of the deoxyribonucleoside triphosphate-synthesizing complex.
|
| |
EMBO J,
18,
1104-1113.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.M.Fox,
F.Maley,
A.Garibian,
L.M.Changchien,
and
P.Van Roey
(1999).
Crystal structure of thymidylate synthase A from Bacillus subtilis.
|
| |
Protein Sci,
8,
538-544.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.Brem,
and
K.A.Dill
(1999).
The effect of multiple binding modes on empirical modeling of ligand docking to proteins.
|
| |
Protein Sci,
8,
1134-1143.
|
 |
|
|
|
|
 |
R.R.Sotelo-Mundo,
J.Ciesla,
J.M.Dzik,
W.Rode,
F.Maley,
G.F.Maley,
L.W.Hardy,
and
W.R.Montfort
(1999).
Crystal structures of rat thymidylate synthase inhibited by Tomudex, a potent anticancer drug.
|
| |
Biochemistry,
38,
1087-1094.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.E.Greasley,
M.M.Yamashita,
H.Cai,
S.J.Benkovic,
D.L.Boger,
and
I.A.Wilson
(1999).
New insights into inhibitor design from the crystal structure and NMR studies of Escherichia coli GAR transformylase in complex with beta-GAR and 10-formyl-5,8,10-trideazafolic acid.
|
| |
Biochemistry,
38,
16783-16793.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Hazebrouck,
F.Maley,
V.Machtelinckx,
P.Sonigo,
and
J.J.Kupiec
(1999).
Structural and functional analysis of surface domains unique to bacteriophage T4 thymidylate synthase.
|
| |
Biochemistry,
38,
2094-2101.
|
 |
|
|
|
|
 |
T.J.Stout,
D.Tondi,
M.Rinaldi,
D.Barlocco,
P.Pecorari,
D.V.Santi,
I.D.Kuntz,
R.M.Stroud,
B.K.Shoichet,
and
M.P.Costi
(1999).
Structure-based design of inhibitors specific for bacterial thymidylate synthase.
|
| |
Biochemistry,
38,
1607-1617.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.P.Lobo,
M.G.Nair,
L.Changchien,
A.Weichsel,
W.R.Montfort,
and
F.Maley
(1998).
Mode of action of site-directed irreversible folate analogue inhibitors of thymidylate synthase.
|
| |
Biochemistry,
37,
4535-4542.
|
 |
|
|
|
|
 |
C.R.Sage,
M.D.Michelitsch,
T.J.Stout,
D.Biermann,
R.Nissen,
J.Finer-Moore,
and
R.M.Stroud
(1998).
D221 in thymidylate synthase controls conformation change, and thereby opening of the imidazolidine.
|
| |
Biochemistry,
37,
13893-13901.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
D.J.Steadman,
P.S.Zhao,
H.T.Spencer,
R.B.Dunlap,
and
S.H.Berger
(1998).
A structural role for glutamine 214 in human thymidylate synthase.
|
| |
Biochemistry,
37,
7089-7095.
|
 |
|
|
|
|
 |
D.M.Landis,
and
L.A.Loeb
(1998).
Random sequence mutagenesis and resistance to 5-fluorouridine in human thymidylate synthases.
|
| |
J Biol Chem,
273,
25809-25817.
|
 |
|
|
|
|
 |
F.Duffieux,
P.Peyret,
B.A.Roe,
and
C.P.Vivares
(1998).
First report on the systematic sequencing of the small genome of Encephalitozoon cuniculi (Protozoa, Microspora): gene organization of a 4.3 kbp region on chromosome I.
|
| |
Microb Comp Genomics,
3,
1.
|
 |
|
|
|
|
 |
M.P.Costi
(1998).
Thymidylate synthase inhibition: a structure-based rationale for drug design.
|
| |
Med Res Rev,
18,
21-42.
|
 |
|
|
|
|
 |
R.Aurora,
and
G.D.Rose
(1998).
Seeking an ancient enzyme in Methanococcus jannaschii using ORF, a program based on predicted secondary structure comparisons.
|
| |
Proc Natl Acad Sci U S A,
95,
2818-2823.
|
 |
|
|
|
|
 |
T.J.Stout,
C.R.Sage,
and
R.M.Stroud
(1998).
The additivity of substrate fragments in enzyme-ligand binding.
|
| |
Structure,
6,
839-848.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.J.Stout,
U.Schellenberger,
D.V.Santi,
and
R.M.Stroud
(1998).
Crystal structures of a unique thermal-stable thymidylate synthase from Bacillus subtilis.
|
| |
Biochemistry,
37,
14736-14747.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
V.Prasanna,
S.Bhattacharjya,
and
P.Balaram
(1998).
Synthetic interface peptides as inactivators of multimeric enzymes: inhibitory and conformational properties of three fragments from Lactobacillus casei thymidylate synthase.
|
| |
Biochemistry,
37,
6883-6893.
|
 |
|
|
|
|
 |
Y.Tong,
X.Liu-Chen,
E.A.Ercikan-Abali,
G.M.Capiaux,
S.C.Zhao,
D.Banerjee,
and
J.R.Bertino
(1998).
Isolation and characterization of thymitaq (AG337) and 5-fluoro-2-deoxyuridylate-resistant mutants of human thymidylate synthase from ethyl methanesulfonate-exposed human sarcoma HT1080 cells.
|
| |
J Biol Chem,
273,
11611-11618.
|
 |
|
|
|
|
 |
Y.Tong,
X.Liu-Chen,
E.A.Ercikan-Abali,
S.C.Zhao,
D.Banerjee,
F.Maley,
and
J.R.Bertino
(1998).
Probing the folate-binding site of human thymidylate synthase by site-directed mutagenesis. Generation of mutants that confer resistance to raltitrexed, Thymitaq, and BW1843U89.
|
| |
J Biol Chem,
273,
31209-31214.
|
 |
|
|
|
|
 |
D.C.Hyatt,
F.Maley,
and
W.R.Montfort
(1997).
Use of strain in a stereospecific catalytic mechanism: crystal structures of Escherichia coli thymidylate synthase bound to FdUMP and methylenetetrahydrofolate.
|
| |
Biochemistry,
36,
4585-4594.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
E.Cheung,
L.D'Ari,
J.C.Rabinowitz,
D.H.Dyer,
J.Y.Huang,
and
B.L.Stoddard
(1997).
Purification, crystallization, and preliminary x-ray studies of a bifunctional 5,10-methenyl/methylene-tetrahydrofolate cyclohydrolase/dehydrogenase from Escherichia coli.
|
| |
Proteins,
27,
322-324.
|
 |
|
|
|
|
 |
H.T.Spencer,
J.E.Villafranca,
and
J.R.Appleman
(1997).
Kinetic scheme for thymidylate synthase from Escherichia coli: determination from measurements of ligand binding, primary and secondary isotope effects, and pre-steady-state catalysis.
|
| |
Biochemistry,
36,
4212-4222.
|
 |
|
|
|
|
 |
K.M.Kerr,
and
L.Hedstrom
(1997).
The roles of conserved carboxylate residues in IMP dehydrogenase and identification of a transition state analog.
|
| |
Biochemistry,
36,
13365-13373.
|
 |
|
|
|
|
 |
P.Strop,
L.Changchien,
F.Maley,
and
W.R.Montfort
(1997).
Crystal structures of a marginally active thymidylate synthase mutant, Arg 126-->Glu.
|
| |
Protein Sci,
6,
2504-2511.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
W.A.Samsonoff,
J.Reston,
M.McKee,
B.O'Connor,
J.Galivan,
G.Maley,
and
F.Maley
(1997).
Intracellular location of thymidylate synthase and its state of phosphorylation.
|
| |
J Biol Chem,
272,
13281-13285.
|
 |
|
|
|
|
 |
W.R.Montfort,
and
A.Weichsel
(1997).
Thymidylate synthase: structure, inhibition, and strained conformations during catalysis.
|
| |
Pharmacol Ther,
76,
29-43.
|
 |
|
|
|
|
 |
B.L.Stoddard
(1996).
Intermediate trapping and laue X-ray diffraction: potential for enzyme mechanism, dynamics, and inhibitor screening.
|
| |
Pharmacol Ther,
70,
215-256.
|
 |
|
|
|
|
 |
C.D.Mol,
J.M.Harris,
E.M.McIntosh,
and
J.A.Tainer
(1996).
Human dUTP pyrophosphatase: uracil recognition by a beta hairpin and active sites formed by three separate subunits.
|
| |
Structure,
4,
1077-1092.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
C.R.Sage,
E.E.Rutenber,
T.J.Stout,
and
R.M.Stroud
(1996).
An essential role for water in an enzyme reaction mechanism: the crystal structure of the thymidylate synthase mutant E58Q.
|
| |
Biochemistry,
35,
16270-16281.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
E.E.Rutenber,
and
R.M.Stroud
(1996).
Binding of the anticancer drug ZD1694 to E. coli thymidylate synthase: assessing specificity and affinity.
|
| |
Structure,
4,
1317-1324.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
I.M.Schlichtherle,
D.S.Roos,
and
J.L.Van Houten
(1996).
Cloning and molecular analysis of the bifunctional dihydrofolate reductase-thymidylate synthase gene in the ciliated protozoan Paramecium tetraurelia.
|
| |
Mol Gen Genet,
250,
665-673.
|
 |
|
|
|
|
 |
J.S.Finer-Moore,
L.Liu,
C.E.Schafmeister,
D.L.Birdsall,
T.Mau,
D.V.Santi,
and
R.M.Stroud
(1996).
Partitioning roles of side chains in affinity, orientation, and catalysis with structures for mutant complexes: asparagine-229 in thymidylate synthase.
|
| |
Biochemistry,
35,
5125-5136.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.M.McGaughey,
L.J.Wheeler,
J.T.Moore,
G.F.Maley,
F.Maley,
and
C.K.Mathews
(1996).
Protein-protein interactions involving T4 phage-coded deoxycytidylate deaminase and thymidylate synthase.
|
| |
J Biol Chem,
271,
23037-23042.
|
 |
|
|
|
|
 |
K.Scheffzek,
W.Kliche,
L.Wiesmüller,
and
J.Reinstein
(1996).
Crystal structure of the complex of UMP/CMP kinase from Dictyostelium discoideum and the bisubstrate inhibitor P1-(5'-adenosyl) P5-(5'-uridyl) pentaphosphate (UP5A) and Mg2+ at 2.2 A: implications for water-mediated specificity.
|
| |
Biochemistry,
35,
9716-9727.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
P.M.Costi,
L.Liu,
J.S.Finer-Moore,
R.M.Stroud,
and
D.V.Santi
(1996).
Asparagine 229 mutants of thymidylate synthase catalyze the methylation of 3-methyl-2'-deoxyuridine 5'-monophosphate.
|
| |
Biochemistry,
35,
3944-3949.
|
 |
|
|
|
|
 |
S.Zhao,
and
S.W.Ragsdale
(1996).
A conformational change in the methyltransferase from Clostridium thermoaceticum facilitates the methyl transfer from (6S)-methyltetrahydrofolate to the corrinoid/iron-sulfur protein in the Acetyl-CoA pathway.
|
| |
Biochemistry,
35,
2476-2481.
|
 |
|
|
|
|
 |
T.J.Stout,
and
R.M.Stroud
(1996).
The complex of the anti-cancer therapeutic, BW1843U89, with thymidylate synthase at 2.0 A resolution: implications for a new mode of inhibition.
|
| |
Structure,
4,
67-77.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Weichsel,
and
W.R.Montfort
(1995).
Ligand-induced distortion of an active site in thymidylate synthase upon binding anticancer drug 1843U89.
|
| |
Nat Struct Biol,
2,
1095-1101.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Weichsel,
W.R.Montfort,
J.Cieśla,
and
F.Maley
(1995).
Promotion of purine nucleotide binding to thymidylate synthase by a potent folate analogue inhibitor, 1843U89.
|
| |
Proc Natl Acad Sci U S A,
92,
3493-3497.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
D.M.Voeller,
L.M.Changchien,
G.F.Maley,
F.Maley,
T.Takechi,
R.E.Turner,
W.R.Montfort,
C.J.Allegra,
and
E.Chu
(1995).
Characterization of a specific interaction between Escherichia coli thymidylate synthase and Escherichia coli thymidylate synthase mRNA.
|
| |
Nucleic Acids Res,
23,
869-875.
|
 |
|
|
|
|
 |
J.T.Kealey,
J.Eckstein,
and
D.V.Santi
(1995).
Role of the conserved tryptophan 82 of Lactobacillus casei thymidylate synthase.
|
| |
Chem Biol,
2,
609-614.
|
 |
|
|
|
|
 |
L.García-Fuentes,
P.Reche,
O.López-Mayorga,
D.V.Santi,
D.González-Pacanowska,
and
C.Barón
(1995).
Thermodynamic analysis of the binding of 5-fluoro-2'-deoxyuridine 5'-monophosphate to thymidylate synthase over a range of temperatures.
|
| |
Eur J Biochem,
232,
641-645.
|
 |
|
|
|
|
 |
P.T.Harrison,
J.E.Scott,
M.J.Hutchinson,
and
R.Thompson
(1995).
Site-directed mutagenesis of varicella-zoster virus thymidylate synthase. Analysis of two highly conserved regions of the enzyme.
|
| |
Eur J Biochem,
230,
511-516.
|
 |
|
|
|
|
 |
R.M.Stroud,
and
E.B.Fauman
(1995).
Significance of structural changes in proteins: expected errors in refined protein structures.
|
| |
Protein Sci,
4,
2392-2404.
|
 |
|
|
|
|
 |
D.R.Knighton,
C.C.Kan,
E.Howland,
C.A.Janson,
Z.Hostomska,
K.M.Welsh,
and
D.A.Matthews
(1994).
Structure of and kinetic channelling in bifunctional dihydrofolate reductase-thymidylate synthase.
|
| |
Nat Struct Biol,
1,
186-194.
|
 |
|
|
|
|
 |
L.W.Hardy,
D.F.Pacitti,
and
E.Nalivaika
(1994).
Use of a purified heterodimer to test negative cooperativity as the basis of substrate inactivation of Escherichia coli thymidylate synthase (Asn177-->Asp).
|
| |
Structure,
2,
833-838.
|
 |
|
|
|
|
 |
N.V.Grishin,
and
M.A.Phillips
(1994).
The subunit interfaces of oligomeric enzymes are conserved to a similar extent to the overall protein sequences.
|
| |
Protein Sci,
3,
2455-2458.
|
 |
|
|
|
|
 |
P.J.Greene,
P.L.Yu,
J.Zhao,
C.A.Schiffer,
and
D.Santi
(1994).
Expression, purification, and characterization of thymidylate synthase from Lactococcus lactis.
|
| |
Protein Sci,
3,
1114-1116.
|
 |
|
|
|
|
 |
R.M.Stroud
(1994).
An electrostatic highway.
|
| |
Nat Struct Biol,
1,
131-134.
|
 |
|
|
|
|
 |
C.A.Orengo,
and
J.M.Thornton
(1993).
Alpha plus beta folds revisited: some favoured motifs.
|
| |
Structure,
1,
105-120.
|
 |
|
|
|
|
 |
L.Liu,
and
D.V.Santi
(1993).
Asparagine 229 in thymidylate synthase contributes to, but is not essential for, catalysis.
|
| |
Proc Natl Acad Sci U S A,
90,
8604-8608.
|
 |
|
|
|
|
 |
P.Schimmell
(1993).
Functional analysis suggests unexpected role for conserved active-site residue in enzyme of known structure.
|
| |
Proc Natl Acad Sci U S A,
90,
9235-9236.
|
 |
|
|
|
|
 |
C.L.Wellington,
and
J.G.Belasco
(1992).
Autoregulation through translation.
|
| |
Curr Biol,
2,
216-218.
|
 |
|
|
|
|
 |
C.W.Kim,
M.L.Michaels,
and
J.H.Miller
(1992).
Amino acid substitution analysis of E. coli thymidylate synthase: the study of a highly conserved region at the N-terminus.
|
| |
Proteins,
13,
352-363.
|
 |
|
|
|
|
 |
D.E.Seitz
(1992).
Oncology drug discovery and clinical trial testing: who's listening?
|
| |
Cancer Invest,
10,
327-329.
|
 |
|
|
|
|
 |
L.W.Hardy,
and
E.Nalivaika
(1992).
Asn177 in Escherichia coli thymidylate synthase is a major determinant of pyrimidine specificity.
|
| |
Proc Natl Acad Sci U S A,
89,
9725-9729.
|
 |
|
|
|
|
 |
M.D.Walkinshaw
(1992).
Protein targets for structure-based drug design.
|
| |
Med Res Rev,
12,
317-372.
|
 |
|
|
|
|
 |
T.Earnest,
E.Fauman,
C.S.Craik,
and
R.Stroud
(1991).
1.59 A structure of trypsin at 120 K: comparison of low temperature and room temperature structures.
|
| |
Proteins,
10,
171-187.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

