Enoyl-[acyl-carrier-protein] reductase (NADPH, B-specific)
2-enoyl thioester reductases catalyse the reduction of trans-2-enoyl acyl carrier protein/coenzyme A to acyl carrier protein/coenzyme A. Yeast enzymes, such as Etr1p and Mrf1p, are responsible for the NADPH dependent reaction in mitochondrial fatty acid synthesis, and are indispensible for respiratory function in yeast. They belong to the medium chain dehydrogenases/reductases (MDR) superfamily and are structurally distinguishable from the prokaryotic 2-enoyl thioester which belong to the short chain dehydrogenases/reductases (SDR) superfamily. The Candida tropicalis genes ETR1 and ETR2 both encode enzymatically active 2-enoyl thioester reductases, which can form both homodimers and heterodimers.
Reference Protein and Structure
- Sequence
-
Q8WZM3
 (1.3.1.104)
(1.3.1.104)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Candida tropicalis (Yeast)

- PDB
-
1guf
- Enoyl thioester reductase from Candida tropicalis
(2.25 Å)



- Catalytic CATH Domains
-
3.90.180.10
 (see all for 1guf)
(see all for 1guf)
Enzyme Reaction (EC:1.3.1.10)
Enzyme Mechanism
Introduction
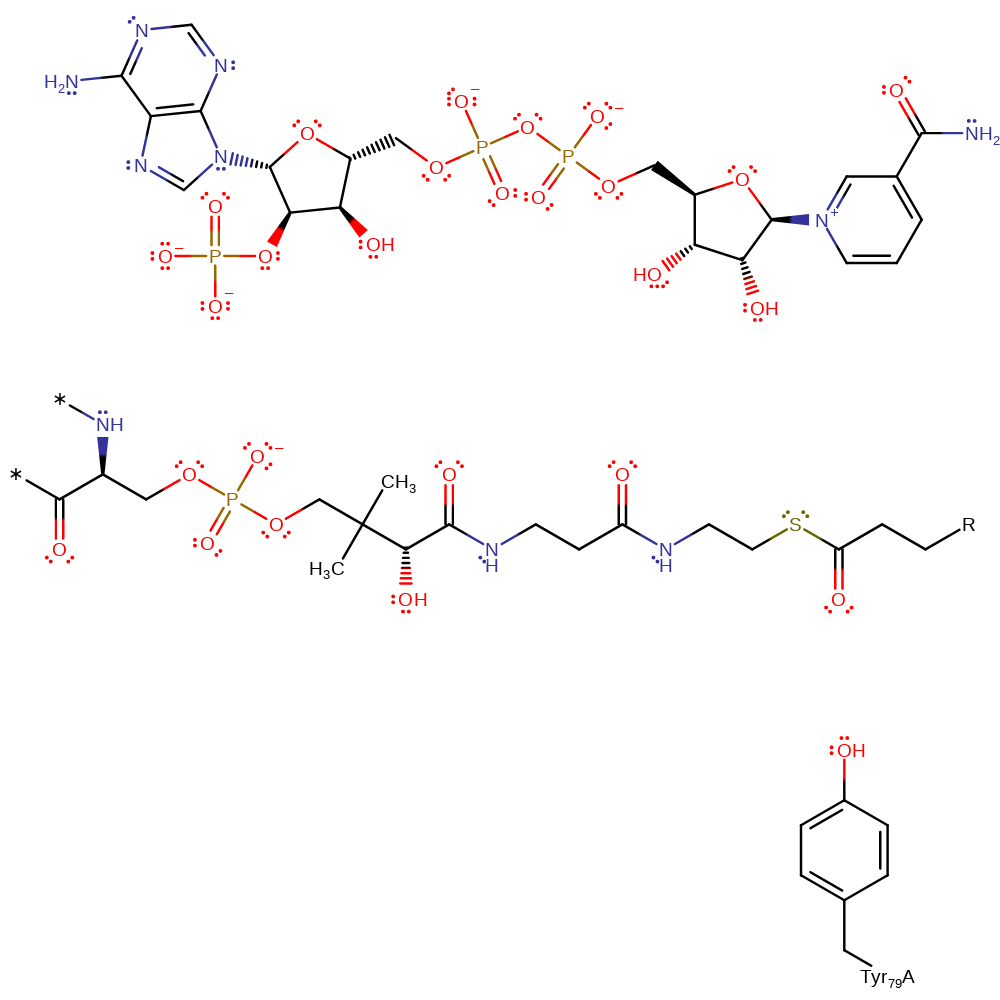
The proposed mechanism is based on analogy with the prokaryotic enoyl thioester reductases such as InhA. A hydride is transferred from NADPH to the beta position of the alpha-beta unsaturated carbonyl substrate (Michael addition) to give an enol(ate) intermediate. The hydroxy group of Tyr 79 acts as an electrophilic catalyst by forming a hydrogen bond to the substrate carbonyl to stabilise the intermediate and the transition state leading to it.
Catalytic Residues Roles
| UniProt | PDB* (1guf) | ||
| Tyr79 | Tyr79(57)A | Hydroxyl group acts as an electrophilic catalyst, forming a hydrogen bond to the carbonyl of the substrate to stabilise the transition state. | increase electrophilicity, electrostatic stabiliser |
Chemical Components
aromatic unimolecular elimination by the conjugate base, michael addition, hydride transfer, overall reactant used, intermediate formation, cofactor used, overall product formed, proton transfer, intermediate collapseReferences
- Airenne TT et al. (2003), J Mol Biol, 327, 47-59. Structure–function Analysis of Enoyl Thioester Reductase Involved in Mitochondrial Maintenance. DOI:10.1016/s0022-2836(03)00038-x. PMID:12614607.
- Parikh S et al. (1999), Biochemistry, 38, 13623-13634. Roles of Tyrosine 158 and Lysine 165 in the Catalytic Mechanism of InhA, the Enoyl-ACP Reductase fromMycobacterium tuberculosis†. DOI:10.1021/bi990529c. PMID:10521269.

Step 1. Tyr79 acts as an electrophilic catalyst by hydrogen bonding to the carbonyl group of alpha-beta unsaturated carbonyl substrate. A hydride is transferred from NADPH to the beta-position in a conjugate addition reaction, forming an intermediate enolate and NADP.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Tyr79(57)A | electrostatic stabiliser |
| Tyr79(57)A | increase electrophilicity |
Chemical Components
ingold: aromatic unimolecular elimination by the conjugate base, michael addition, hydride transfer, overall reactant used, intermediate formation, cofactor used
Step 2. The enolate intermediate collapses and the substrate is protonated at the alpha-carbon, forming the reduced product.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Tyr79(57)A | electrostatic stabiliser |





 Download:
Download: