Protein histidine kinase (CheA)
Chemotaxis Receptor A (CheA) is involved in the cytoplasmic phosphorylation cascade that couples changes in bacterial swimming behaviour to the external concentrations of attractants and repellents. CheA catalyses the trans-phosphorylation of CheY to generate intracellular signals between the membrane-spanning chemoreceptors and the flagellar motor. The CheA homodimer responds to asymmetric activation. Although there are two ATP-binding sites, one per CheA dimer, only a single binding domain at any given time can participate in catalysis. An aspartate reside of the chemoreceptor CheY is specifically phosphorylated as part of the chemotaxis cascade.
Reference Protein and Structure
- Sequence
-
Q56310
 (2.7.13.3)
(2.7.13.3)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Thermotoga maritima MSB8 (Bacteria)

- PDB
-
1i58
- STRUCTURE OF THE HISTIDINE KINASE CHEA ATP-BINDING DOMAIN IN COMPLEX WITH ATP ANALOG ADPCP AND MAGNESIUM
(1.6 Å)



- Catalytic CATH Domains
-
3.30.565.10
 (see all for 1i58)
(see all for 1i58)
- Cofactors
- Magnesium(2+) (1) Metal MACiE
Enzyme Mechanism
Introduction
This kinase first phosphorylates His45, the phosphate group is then passed on to the nucleophilic Asp of the CheY substrate.
Catalytic Residues Roles
| UniProt | PDB* (1i58) | ||
| Asn409 | Asn409(58)A | Helps orient the ATP substrate, activating and stabilising it during the initial phosphorylation of the nucleophilic histidine residue and binds Mg2+ ion. | hydrogen bond donor, metal ligand, electrostatic stabiliser, steric role |
| His45 | Not found | Acts as a nucleophile, abstracting the terminal phosphate group from the ATP substrate and passing it onto the aspartate residue of the CheY substrate. | covalently attached, hydrogen bond donor, nucleophile, nucleofuge, activator |
| Glu67 | Not found | Activates the nucleophilic histidine residue. | increase nucleophilicity, hydrogen bond acceptor, electrostatic stabiliser |
Chemical Components
bimolecular nucleophilic substitution, intermediate formation, overall product formed, overall reactant used, enzyme-substrate complex formation, intermediate collapse, dephosphorylation, enzyme-substrate complex cleavage, native state of enzyme regenerated, inferred reaction stepReferences
- Levit MN et al. (1999), Biochemistry, 38, 6651-6658. Mechanism of CheA Protein Kinase Activation in Receptor Signaling Complexes†. DOI:10.1021/bi982839l. PMID:10350484.
- Shi T et al. (2011), J Phys Chem B, 115, 11895-11901. Mechanism for the autophosphorylation of CheA histidine kinase: QM/MM calculations. DOI:10.1021/jp203968d. PMID:21910494.
- Zhang J et al. (2005), J Am Chem Soc, 127, 11709-11719. Dynamic Mechanism for the Autophosphorylation of CheA Histidine Kinase: Molecular Dynamics Simulations. DOI:10.1021/ja051199o. PMID:16104748.
- Bilwes AM et al. (2001), Nat Struct Biol, 8, 353-360. Nucleotide binding by the histidine kinase CheA. DOI:10.1038/86243. PMID:11276258.
- Bilwes AM et al. (1999), Cell, 96, 131-141. Structure of CheA, a Signal-Transducing Histidine Kinase. DOI:10.1016/s0092-8674(00)80966-6. PMID:9989504.
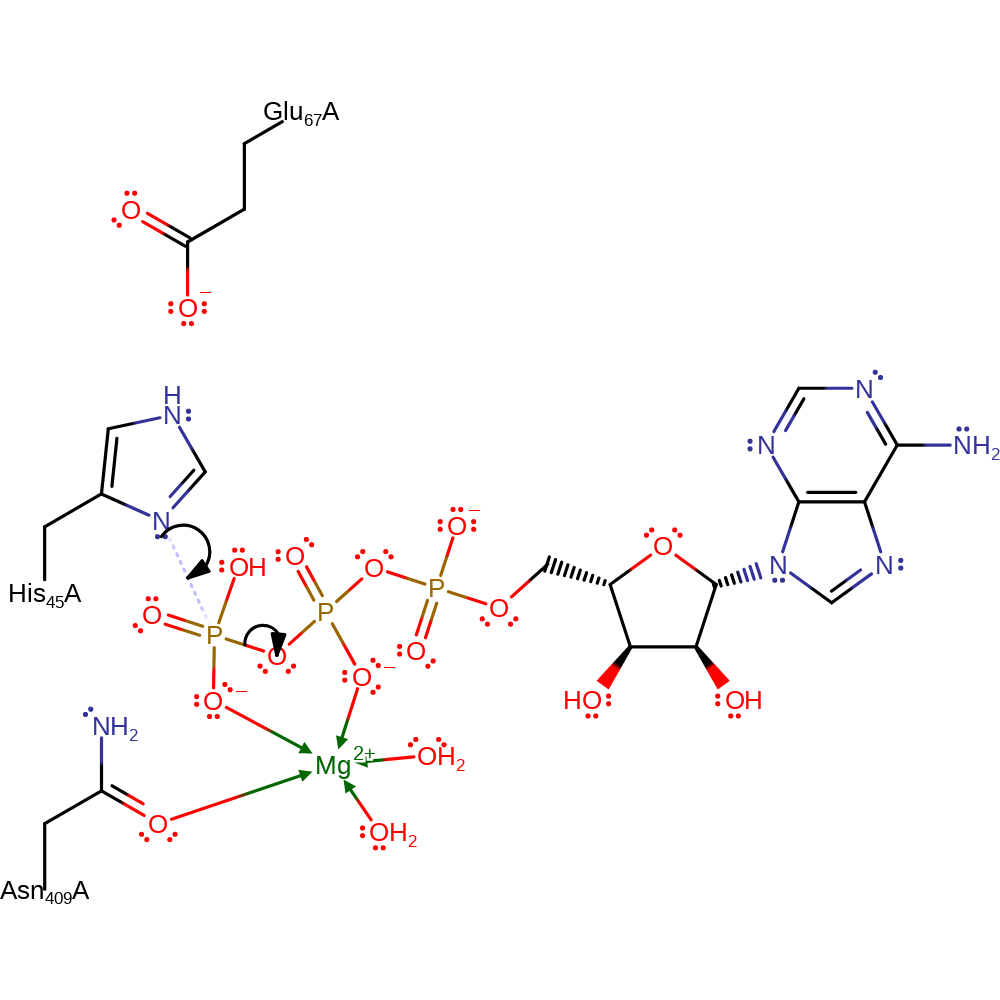
Step 1. His45, activated by Glu67, acts as a nucleophile towards the terminal phosphate group of ATP.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn409(58)A | hydrogen bond donor, electrostatic stabiliser, steric role |
| His45 | activator, hydrogen bond donor, covalently attached |
| Glu67 | electrostatic stabiliser, increase nucleophilicity |
| Asn409(58)A | metal ligand |
| His45 | nucleophile |
Chemical Components
ingold: bimolecular nucleophilic substitution, intermediate formation, overall product formed, overall reactant used, enzyme-substrate complex formation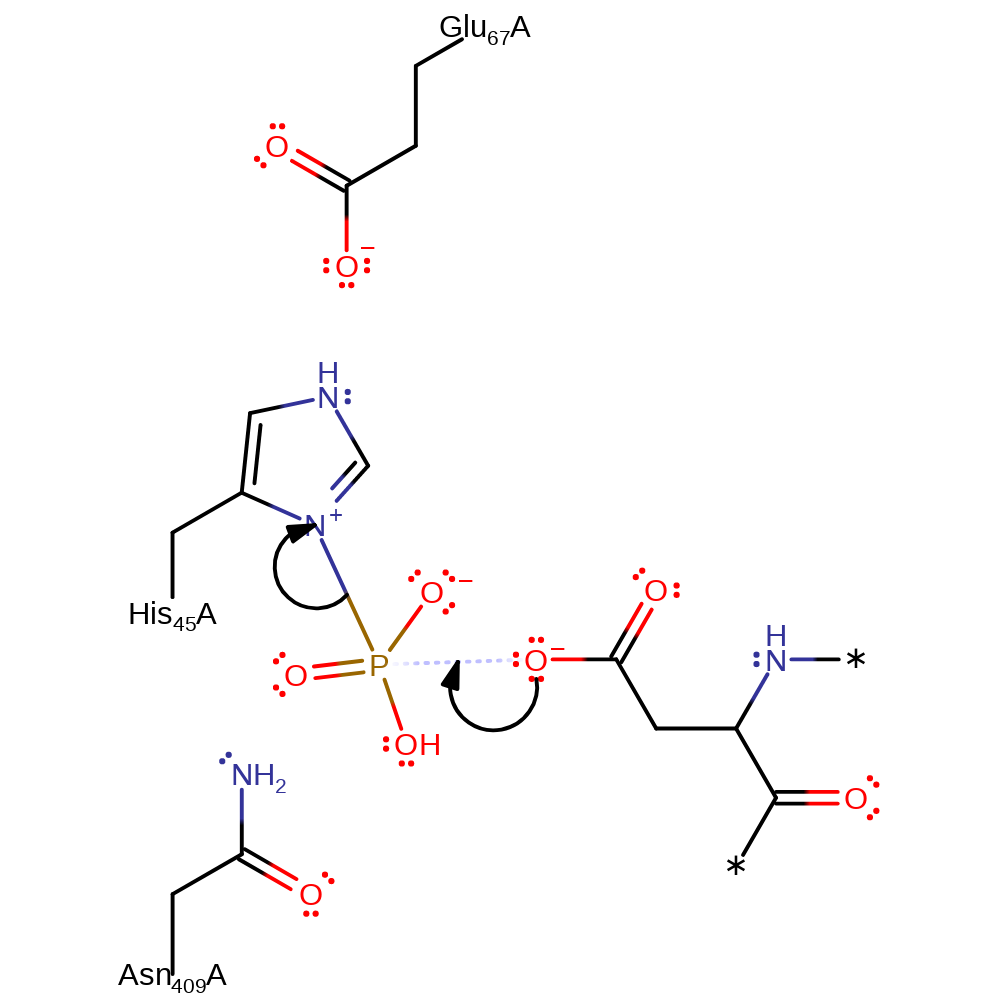
Step 2. The nucleophilic Asp of chemoreceptor Y (CheY) attacks the phosphorylated His45, removing the functional group and regenerating the active site.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His45 | activator, hydrogen bond donor |
| Glu67 | hydrogen bond acceptor |
| His45 | nucleofuge |




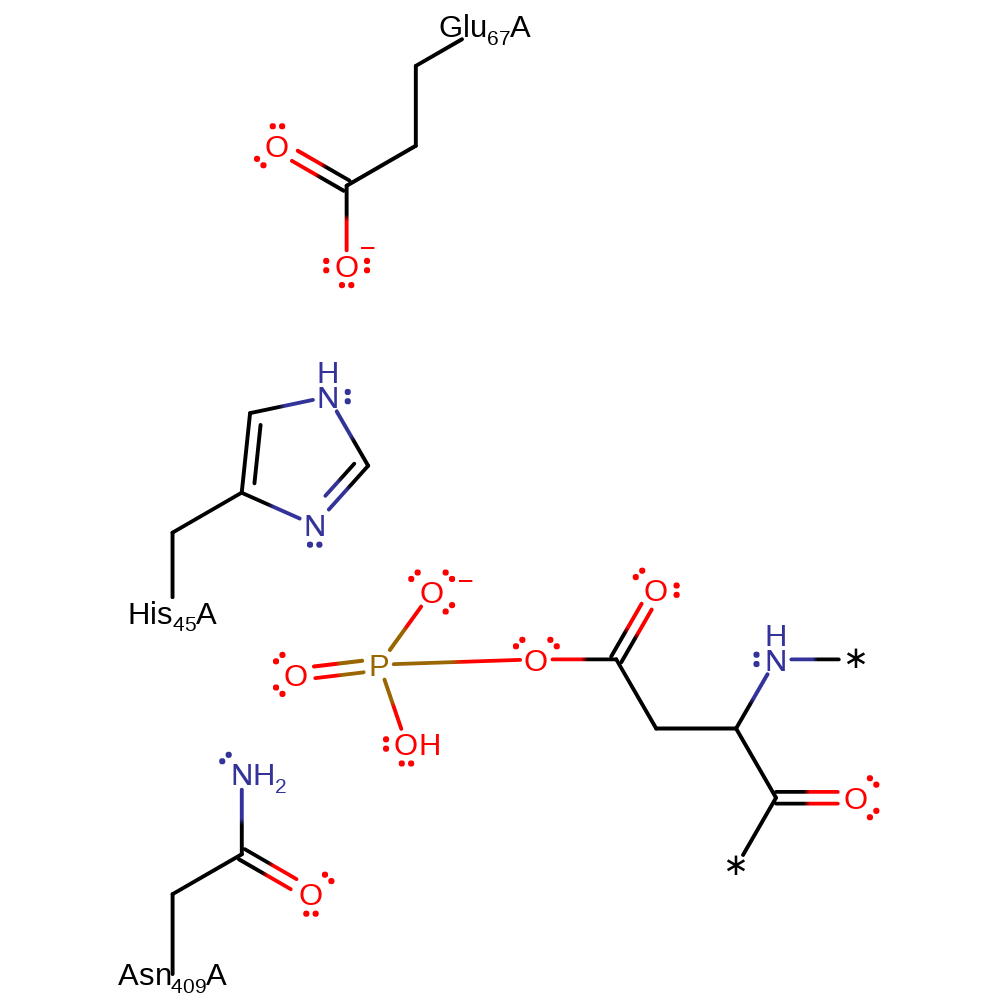 Download:
Download: