 |
PDBsum entry 1h5c

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1h5c
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.11.1.7
- peroxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
2 a phenolic donor + H2O2 = 2 a phenolic radical donor + 2 H2O
|
 |
 |
 |
 |
 |
2
×
a phenolic donor
|
+
|
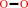 H2O2
H2O2
|
=
|
2
×
a phenolic radical donor
|
+
|
2
×
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Heme
|
 |
 |
 |
 |
 |
Heme
Bound ligand (Het Group name =
HEM)
matches with 95.45% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Nature
417:463-468
(2002)
|
|
PubMed id:
|
|
|
|

|
| |
|
The catalytic pathway of horseradish peroxidase at high resolution.
|
|
G.I.Berglund,
G.H.Carlsson,
A.T.Smith,
H.Szöke,
A.Henriksen,
J.Hajdu.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
A molecular description of oxygen and peroxide activation in biological systems
is difficult, because electrons liberated during X-ray data collection reduce
the active centres of redox enzymes catalysing these reactions. Here we describe
an effective strategy to obtain crystal structures for high-valency redox
intermediates and present a three-dimensional movie of the X-ray-driven
catalytic reduction of a bound dioxygen species in horseradish peroxidase (HRP).
We also describe separate experiments in which high-resolution structures could
be obtained for all five oxidation states of HRP, showing such structures with
preserved redox states for the first time.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1: The five oxidation states of horseradish peroxidase.
|
 |
Figure 4.
Figure 4: Spectra and refined high-resolution structures for the
five oxidation states of horseradish peroxidase. Accession
codes are shown. The structures were captured at high
concentrations in individual experiments (Methods) and were
obtained from the first few degrees of data (Table 2 of the
Supplementary Information). a, Ferric enzyme. b, Ferrous enzyme.
c, Compound III (Fe -O bond distance, 1.8 ┼). d, Compound I (Fe
-O bond distance, 1.7 ┼). e, Compound II (Fe -O bond distance,
1.8 ┼). SigmaA-weighted^30 2mF[obs] -DF[calc] maps contoured at
1  . .
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Macmillan Publishers Ltd:
Nature
(2002,
417,
463-468)
copyright 2002.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.M.Orville,
R.Buono,
M.Cowan,
A.Héroux,
G.Shea-McCarthy,
D.K.Schneider,
J.M.Skinner,
M.J.Skinner,
D.Stoner-Ma,
and
R.M.Sweet
(2011).
Correlated single-crystal electronic absorption spectroscopy and X-ray crystallography at NSLS beamline X26-C.
|
| |
J Synchrotron Radiat,
18,
358-366.
|
 |
|
|
|
|
 |
C.Homer,
L.Cooper,
and
A.Gonzalez
(2011).
Energy dependence of site-specific radiation damage in protein crystals.
|
| |
J Synchrotron Radiat,
18,
338-345.
|
 |
|
|
|
|
 |
D.H.Juers,
and
M.Weik
(2011).
Similarities and differences in radiation damage at 100 K versus 160 K in a crystal of thermolysin.
|
| |
J Synchrotron Radiat,
18,
329-337.
|
 |
|
|
|
|
 |
R.L.Owen,
B.A.Yorke,
J.A.Gowdy,
and
A.R.Pearson
(2011).
Revealing low-dose radiation damage using single-crystal spectroscopy.
|
| |
J Synchrotron Radiat,
18,
367-373.
|
 |
|
|
|
|
 |
T.M.Cheng,
S.J.Mao,
S.T.Lai,
C.C.Chang,
M.C.Yang,
N.C.Chen,
S.C.Chou,
and
J.P.Pan
(2011).
Haemoglobin-induced oxidative stress is associated with both endogenous peroxidase activity and H(2)O(2) generation from polyunsaturated fatty acids.
|
| |
Free Radic Res,
45,
303-316.
|
 |
|
|
|
|
 |
Y.Wang,
and
Y.Hasebe
(2011).
Carbon-felt-based bioelectrocatalytic flow-detectors: optimization of the adsorption conditions of horseradish peroxidase and thionine onto carbon-felt for highly sensitive amperometric determination of H2O2.
|
| |
Anal Sci,
27,
401.
|
 |
|
|
|
|
 |
C.Hu,
C.D.Sulok,
F.Paulat,
N.Lehnert,
A.I.Twigg,
M.P.Hendrich,
C.E.Schulz,
and
W.R.Scheidt
(2010).
Just a proton: distinguishing the two electronic states of five-coordinate high-spin iron(II) porphyrinates with imidazole/ate coordination.
|
| |
J Am Chem Soc,
132,
3737-3750.
|
 |
|
|
|
|
 |
E.F.Garman
(2010).
Radiation damage in macromolecular crystallography: what is it and why should we care?
|
| |
Acta Crystallogr D Biol Crystallogr,
66,
339-351.
|
 |
|
|
|
|
 |
J.J.Agresti,
E.Antipov,
A.R.Abate,
K.Ahn,
A.C.Rowat,
J.C.Baret,
M.Marquez,
A.M.Klibanov,
A.D.Griffiths,
and
D.A.Weitz
(2010).
Ultrahigh-throughput screening in drop-based microfluidics for directed evolution.
|
| |
Proc Natl Acad Sci U S A,
107,
4004-4009.
|
 |
|
|
|
|
 |
M.Schiltz,
and
G.Bricogne
(2010).
;Broken symmetries' in macromolecular crystallography: phasing from unmerged data.
|
| |
Acta Crystallogr D Biol Crystallogr,
66,
447-457.
|
 |
|
|
|
|
 |
M.Weik,
and
J.P.Colletier
(2010).
Temperature-dependent macromolecular X-ray crystallography.
|
| |
Acta Crystallogr D Biol Crystallogr,
66,
437-446.
|
 |
|
|
|
|
 |
P.Carpentier,
A.Royant,
M.Weik,
and
D.Bourgeois
(2010).
Raman-assisted crystallography suggests a mechanism of X-ray-induced disulfide radical formation and reparation.
|
| |
Structure,
18,
1410-1419.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Westenhoff,
E.Nazarenko,
E.Malmerberg,
J.Davidsson,
G.Katona,
and
R.Neutze
(2010).
Time-resolved structural studies of protein reaction dynamics: a smorgasbord of X-ray approaches.
|
| |
Acta Crystallogr A,
66,
207-219.
|
 |
|
|
|
|
 |
Y.Yoshioka,
and
M.Mitani
(2010).
B3LYP study on reduction mechanisms from O2 to H2O at the catalytic sites of fully reduced and mixed-valence bovine cytochrome c oxidases.
|
| |
Bioinorg Chem Appl,
(),
182804.
|
 |
|
|
|
|
 |
Z.Chen,
L.Xu,
Y.Liang,
and
M.Zhao
(2010).
pH-Sensitive Water-Soluble Nanospheric Imprinted Hydrogels Prepared as Horseradish Peroxidase Mimetic Enzymes.
|
| |
Adv Mater,
22,
1488-1492.
|
 |
|
|
|
|
 |
B.R.Goblirsch,
B.R.Streit,
J.L.DuBois,
and
C.M.Wilmot
(2009).
Crystallization and preliminary X-ray diffraction of chlorite dismutase from Dechloromonas aromatica RCB.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
65,
818-821.
|
 |
|
|
|
|
 |
B.Sjöblom,
M.Polentarutti,
and
K.Djinovic-Carugo
(2009).
Structural study of X-ray induced activation of carbonic anhydrase.
|
| |
Proc Natl Acad Sci U S A,
106,
10609-10613.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
H.Aoyama,
K.Muramoto,
K.Shinzawa-Itoh,
K.Hirata,
E.Yamashita,
T.Tsukihara,
T.Ogura,
and
S.Yoshikawa
(2009).
A peroxide bridge between Fe and Cu ions in the O2 reduction site of fully oxidized cytochrome c oxidase could suppress the proton pump.
|
| |
Proc Natl Acad Sci U S A,
106,
2165-2169.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.Kulys,
Z.Dapkunas,
and
R.Stupak
(2009).
Intensification of biocatalytical processes by synergistic substrate conversion. Fungal peroxidase catalyzed N-hydroxy derivative oxidation in presence of 10-propyl sulfonic acid phenoxazine.
|
| |
Appl Biochem Biotechnol,
158,
445-456.
|
 |
|
|
|
|
 |
K.Marjamaa,
E.M.Kukkola,
and
K.V.Fagerstedt
(2009).
The role of xylem class III peroxidases in lignification.
|
| |
J Exp Bot,
60,
367-376.
|
 |
|
|
|
|
 |
L.Fruk,
J.Kuhlmann,
and
C.M.Niemeyer
(2009).
Analysis of heme-reconstitution of apoenzymes by means of surface plasmon resonance.
|
| |
Chem Commun (Camb),
(),
230-232.
|
 |
|
|
|
|
 |
L.Qiang,
and
J.Zhou
(2009).
Determination of nitric oxide using horseradish peroxidase by UV second-order derivative spectrometry.
|
| |
Anal Sci,
25,
1467-1470.
|
 |
|
|
|
|
 |
R.L.Owen,
A.R.Pearson,
A.Meents,
P.Boehler,
V.Thominet,
and
C.Schulze-Briese
(2009).
A new on-axis multimode spectrometer for the macromolecular crystallography beamlines of the Swiss Light Source.
|
| |
J Synchrotron Radiat,
16,
173-182.
|
 |
|
|
|
|
 |
S.P.de Visser,
L.Tahsini,
and
W.Nam
(2009).
How does the axial ligand of cytochrome P450 biomimetics influence the regioselectivity of aliphatic versus aromatic hydroxylation?
|
| |
Chemistry,
15,
5577-5587.
|
 |
|
|
|
|
 |
X.Zhao,
S.Yu,
K.Ranguelova,
J.Suarez,
L.Metlitsky,
J.P.Schelvis,
and
R.S.Magliozzo
(2009).
Role of the Oxyferrous Heme Intermediate and Distal Side Adduct Radical in the Catalase Activity of Mycobacterium tuberculosis KatG Revealed by the W107F Mutant.
|
| |
J Biol Chem,
284,
7030-7037.
|
 |
|
|
|
|
 |
C.D.Georgiou,
I.Papapostolou,
and
K.Grintzalis
(2008).
Superoxide radical detection in cells, tissues, organisms (animals, plants, insects, microorganisms) and soils.
|
| |
Nat Protoc,
3,
1679-1692.
|
 |
|
|
|
|
 |
C.Hu,
B.C.Noll,
C.E.Schulz,
and
W.R.Scheidt
(2008).
Hydrogen bonding influence of 1,10-phenanthroline on five-coordinate high-spin imidazole-ligated iron(II) porphyrinates.
|
| |
Inorg Chem,
47,
8884-8895.
|
 |
|
|
|
|
 |
C.Hu,
B.C.Noll,
P.M.Piccoli,
A.J.Schultz,
C.E.Schulz,
and
W.R.Scheidt
(2008).
Hydrogen bonding effects on the electronic configuration of five-coordinate high-spin iron(II) porphyrinates.
|
| |
J Am Chem Soc,
130,
3127-3136.
|
 |
|
|
|
|
 |
D.S.Berkholz,
H.R.Faber,
S.N.Savvides,
and
P.A.Karplus
(2008).
Catalytic cycle of human glutathione reductase near 1 A resolution.
|
| |
J Mol Biol,
382,
371-384.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
F.Gruia,
M.Kubo,
X.Ye,
D.Ionascu,
C.Lu,
R.K.Poole,
S.R.Yeh,
and
P.M.Champion
(2008).
Coherence spectroscopy investigations of the low-frequency vibrations of heme: effects of protein-specific perturbations.
|
| |
J Am Chem Soc,
130,
5231-5244.
|
 |
|
|
|
|
 |
F.Wei,
J.Wang,
W.Liao,
B.G.Zimmermann,
D.T.Wong,
and
C.M.Ho
(2008).
Electrochemical detection of low-copy number salivary RNA based on specific signal amplification with a hairpin probe.
|
| |
Nucleic Acids Res,
36,
e65.
|
 |
|
|
|
|
 |
M.Jormakka,
K.Yokoyama,
T.Yano,
M.Tamakoshi,
S.Akimoto,
T.Shimamura,
P.Curmi,
and
S.Iwata
(2008).
Molecular mechanism of energy conservation in polysulfide respiration.
|
| |
Nat Struct Mol Biol,
15,
730-737.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Newcomb,
J.A.Halgrimson,
J.H.Horner,
E.C.Wasinger,
L.X.Chen,
and
S.G.Sligar
(2008).
X-ray absorption spectroscopic characterization of a cytochrome P450 compound II derivative.
|
| |
Proc Natl Acad Sci U S A,
105,
8179-8184.
|
 |
|
|
|
|
 |
R.Davydov,
and
B.M.Hoffman
(2008).
EPR and ENDOR studies of Fe(II) hemoproteins reduced and oxidized at 77 K.
|
| |
J Biol Inorg Chem,
13,
357-369.
|
 |
|
|
|
|
 |
D.M.Hushpulian,
A.A.Poloznikov,
P.A.Savitski,
A.M.Rozhkova,
T.A.Chubar,
V.A.Fechina,
M.A.Orlova,
V.I.Tishkov,
I.G.Gazaryan,
and
L.M.Lagrimini
(2007).
Glutamic acid-141: a heme 'bodyguard' in anionic tobacco peroxidase.
|
| |
Biol Chem,
388,
373-380.
|
 |
|
|
|
|
 |
E.F.Garman,
and
S.M.McSweeney
(2007).
Progress in research into radiation damage in cryo-cooled macromolecular crystals.
|
| |
J Synchrotron Radiat,
14,
1-3.
|
 |
|
|
|
|
 |
G.Katona,
P.Carpentier,
V.Nivière,
P.Amara,
V.Adam,
J.Ohana,
N.Tsanov,
and
D.Bourgeois
(2007).
Raman-assisted crystallography reveals end-on peroxide intermediates in a nonheme iron enzyme.
|
| |
Science,
316,
449-453.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
H.P.Hersleth,
T.Uchida,
A.K.Røhr,
T.Teschner,
V.Schünemann,
T.Kitagawa,
A.X.Trautwein,
C.H.Görbitz,
and
K.K.Andersson
(2007).
Crystallographic and spectroscopic studies of peroxide-derived myoglobin compound II and occurrence of protonated FeIV O.
|
| |
J Biol Chem,
282,
23372-23386.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
I.G.Denisov,
D.C.Victoria,
and
S.G.Sligar
(2007).
Cryoradiolytic reduction of heme proteins: Maximizing dose dependent yield.
|
| |
Radiat Phys Chem Oxf Engl 1993,
76,
714-721.
|
 |
|
|
|
|
 |
K.Kühnel,
E.Derat,
J.Terner,
S.Shaik,
and
I.Schlichting
(2007).
Structure and quantum chemical characterization of chloroperoxidase compound 0, a common reaction intermediate of diverse heme enzymes.
|
| |
Proc Natl Acad Sci U S A,
104,
99.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
L.Huang,
and
P.R.Ortiz de Montellano
(2007).
Arthromyces ramosus peroxidase produces two chlorinating species.
|
| |
Biochem Biophys Res Commun,
355,
581-586.
|
 |
|
|
|
|
 |
L.M.Colosi,
Q.Huang,
and
W.J.Weber
(2007).
Validation of a two-parameter quantitative structure-activity relationship as a legitimate tool for rational re-design of horseradish peroxidase.
|
| |
Biotechnol Bioeng,
98,
295-299.
|
 |
|
|
|
|
 |
M.A.Carrondo,
I.Bento,
P.M.Matias,
and
P.F.Lindley
(2007).
Crystallographic evidence for dioxygen interactions with iron proteins.
|
| |
J Biol Inorg Chem,
12,
429-442.
|
 |
|
|
|
|
 |
M.C.Corbett,
M.J.Latimer,
T.L.Poulos,
I.F.Sevrioukova,
K.O.Hodgson,
and
B.Hedman
(2007).
Photoreduction of the active site of the metalloprotein putidaredoxin by synchrotron radiation.
|
| |
Acta Crystallogr D Biol Crystallogr,
63,
951-960.
|
 |
|
|
|
|
 |
N.Mogharrab,
H.Ghourchian,
and
M.Amininasab
(2007).
Structural stabilization and functional improvement of horseradish peroxidase upon modification of accessible lysines: experiments and simulation.
|
| |
Biophys J,
92,
1192-1203.
|
 |
|
|
|
|
 |
P.Ascenzi,
A.Bocedi,
G.Antonini,
M.Bolognesi,
and
M.Fasano
(2007).
Reductive nitrosylation and peroxynitrite-mediated oxidation of heme-hemopexin.
|
| |
FEBS J,
274,
551-562.
|
 |
|
|
|
|
 |
P.F.Widboom,
E.N.Fielding,
Y.Liu,
and
S.D.Bruner
(2007).
Structural basis for cofactor-independent dioxygenation in vancomycin biosynthesis.
|
| |
Nature,
447,
342-345.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
P.Nicholls
(2007).
The oxygenase-peroxidase theory of Bach and Chodat and its modern equivalents: change and permanence in scientific thinking as shown by our understanding of the roles of water, peroxide, and oxygen in the functioning of redox enzymes.
|
| |
Biochemistry (Mosc),
72,
1039-1046.
|
 |
|
|
|
|
 |
P.R.Rich,
and
M.Iwaki
(2007).
A comparison of catalytic site intermediates of cytochrome c oxidase and peroxidases.
|
| |
Biochemistry (Mosc),
72,
1047-1055.
|
 |
|
|
|
|
 |
R.F.Abdelhamid,
Y.Obara,
Y.Uchida,
T.Kohzuma,
D.M.Dooley,
D.E.Brown,
and
H.Hori
(2007).
Pi-pi interaction between aromatic ring and copper-coordinated His81 imidazole regulates the blue copper active-site structure.
|
| |
J Biol Inorg Chem,
12,
165-173.
|
 |
|
|
|
|
 |
S.Ichimura,
T.Uchida,
S.Taniguchi,
S.Hira,
T.Tosha,
I.Morishima,
T.Kitagawa,
and
K.Ishimori
(2007).
Unique peroxidase reaction mechanism in prostaglandin endoperoxide H synthase-2: compound I in prostaglandin endoperoxide H synthase-2 can be formed without assistance by distal glutamine residue.
|
| |
J Biol Chem,
282,
16681-16690.
|
 |
|
|
|
|
 |
V.de Serrano,
Z.Chen,
M.F.Davis,
and
S.Franzen
(2007).
X-ray crystal structural analysis of the binding site in the ferric and oxyferrous forms of the recombinant heme dehaloperoxidase cloned from Amphitrite ornata.
|
| |
Acta Crystallogr D Biol Crystallogr,
63,
1094-1101.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
Z.H.Wang,
Y.W.Lin,
F.I.Rosell,
F.Y.Ni,
H.J.Lu,
P.Y.Yang,
X.S.Tan,
X.Y.Li,
Z.X.Huang,
and
A.G.Mauk
(2007).
Converting cytochrome C into a peroxidase-like metalloenzyme by molecular design.
|
| |
Chembiochem,
8,
607-609.
|
 |
|
|
|
|
 |
B.Kauffmann,
M.S.Weiss,
V.S.Lamzin,
and
A.Schmidt
(2006).
How to avoid premature decay of your macromolecular crystal: a quick soak for long life.
|
| |
Structure,
14,
1099-1105.
|
 |
|
|
|
|
 |
B.U.Klink,
R.S.Goody,
and
A.J.Scheidig
(2006).
A newly designed microspectrofluorometer for kinetic studies on protein crystals in combination with x-ray diffraction.
|
| |
Biophys J,
91,
981-992.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
E.F.Garman,
and
R.L.Owen
(2006).
Cryocooling and radiation damage in macromolecular crystallography.
|
| |
Acta Crystallogr D Biol Crystallogr,
62,
32-47.
|
 |
|
|
|
|
 |
G.Battistuzzi,
M.Bellei,
F.De Rienzo,
and
M.Sola
(2006).
Redox properties of the Fe3+/Fe2+ couple in Arthromyces ramosus class II peroxidase and its cyanide adduct.
|
| |
J Biol Inorg Chem,
11,
586-592.
|
 |
|
|
|
|
 |
G.Zoppellaro,
T.Teschner,
E.Harbitz,
V.Schünemann,
S.Karlsen,
D.M.Arciero,
S.Ciurli,
A.X.Trautwein,
A.B.Hooper,
and
K.K.Andersson
(2006).
Low-temperature EPR and Mössbauer spectroscopy of two cytochromes with His-Met axial coordination exhibiting HALS signals.
|
| |
Chemphyschem,
7,
1258-1267.
|
 |
|
|
|
|
 |
H.K.Leiros,
J.Timmins,
R.B.Ravelli,
and
S.M.McSweeney
(2006).
Is radiation damage dependent on the dose rate used during macromolecular crystallography data collection?
|
| |
Acta Crystallogr D Biol Crystallogr,
62,
125-132.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.C.Thomas,
C.Pacholski,
and
M.J.Sailor
(2006).
Delivery of nanogram payloads using magnetic porous silicon microcarriers.
|
| |
Lab Chip,
6,
782-787.
|
 |
|
|
|
|
 |
J.N.Harvey,
C.M.Bathelt,
and
A.J.Mulholland
(2006).
QM/MM modeling of compound I active species in cytochrome P450, cytochrome C peroxidase, and ascorbate peroxidase.
|
| |
J Comput Chem,
27,
1352-1362.
|
 |
|
|
|
|
 |
K.L.Stone,
R.K.Behan,
and
M.T.Green
(2006).
Resonance Raman spectroscopy of chloroperoxidase compound II provides direct evidence for the existence of an iron(IV)-hydroxide.
|
| |
Proc Natl Acad Sci U S A,
103,
12307-12310.
|
 |
|
|
|
|
 |
L.Fruk,
J.Müller,
and
C.M.Niemeyer
(2006).
Kinetic analysis of semisynthetic peroxidase enzymes containing a covalent DNA-heme adduct as the cofactor.
|
| |
Chemistry,
12,
7448-7457.
|
 |
|
|
|
|
 |
M.Grabolle,
M.Haumann,
C.Müller,
P.Liebisch,
and
H.Dau
(2006).
Rapid loss of structural motifs in the manganese complex of oxygenic photosynthesis by X-ray irradiation at 10-300 K.
|
| |
J Biol Chem,
281,
4580-4588.
|
 |
|
|
|
|
 |
S.K.Badyal,
M.G.Joyce,
K.H.Sharp,
H.E.Seward,
M.Mewies,
J.Basran,
I.K.Macdonald,
P.C.Moody,
and
E.L.Raven
(2006).
Conformational mobility in the active site of a heme peroxidase.
|
| |
J Biol Chem,
281,
24512-24520.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.P.de Visser
(2006).
Differences in and comparison of the catalytic properties of heme and non-heme enzymes with a central oxo-iron group.
|
| |
Angew Chem Int Ed Engl,
45,
1790-1793.
|
 |
|
|
|
|
 |
T.Prieto,
R.O.Marcon,
F.M.Prado,
A.C.Caires,
P.Di Mascio,
S.Brochsztain,
O.R.Nascimento,
and
I.L.Nantes
(2006).
Reaction route control by microperoxidase-9/CTAB micelle ratios.
|
| |
Phys Chem Chem Phys,
8,
1963-1973.
|
 |
|
|
|
|
 |
W.Wu,
J.Lu,
Y.Wei,
J.Wang,
J.Lin,
S.Cao,
X.Sun,
and
K.Tang
(2006).
Isolation and characterization of the first putative peroxidase gene from oilseed rape (Brassica napus) which is highly homologous to HRPC.
|
| |
Biosci Rep,
26,
263-280.
|
 |
|
|
|
|
 |
X.Yan,
X.Shi,
K.Hill,
and
H.F.Ji
(2006).
Microcantilevers modified by horseradish peroxidase intercalated nano-assembly for hydrogen peroxide detection.
|
| |
Anal Sci,
22,
205-208.
|
 |
|
|
|
|
 |
A.P.Dubnovitsky,
R.B.Ravelli,
A.N.Popov,
and
A.C.Papageorgiou
(2005).
Strain relief at the active site of phosphoserine aminotransferase induced by radiation damage.
|
| |
Protein Sci,
14,
1498-1507.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Schmidt,
and
V.S.Lamzin
(2005).
Extraction of functional motion in trypsin crystal structures.
|
| |
Acta Crystallogr D Biol Crystallogr,
61,
1132-1139.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
B.D.Howes,
N.C.Brissett,
W.A.Doyle,
A.T.Smith,
and
G.Smulevich
(2005).
Spectroscopic and kinetic properties of the horseradish peroxidase mutant T171S. Evidence for selective effects on the reduced state of the enzyme.
|
| |
FEBS J,
272,
5514-5521.
|
 |
|
|
|
|
 |
C.Rovira
(2005).
Structure, protonation state and dynamics of catalase compound II.
|
| |
Chemphyschem,
6,
1820-1826.
|
 |
|
|
|
|
 |
D.Bourgeois,
and
A.Royant
(2005).
Advances in kinetic protein crystallography.
|
| |
Curr Opin Struct Biol,
15,
538-547.
|
 |
|
|
|
|
 |
D.Kumar,
S.P.de Visser,
P.K.Sharma,
E.Derat,
and
S.Shaik
(2005).
The intrinsic axial ligand effect on propene oxidation by horseradish peroxidase versus cytochrome P450 enzymes.
|
| |
J Biol Inorg Chem,
10,
181-189.
|
 |
|
|
|
|
 |
L.G.Fenoll,
F.García-Molina,
M.A.Gilabert,
R.Varón,
P.A.García-Ruiz,
J.Tudela,
F.García-Cánovas,
and
J.N.Rodríguez-López
(2005).
Interpretation of the reactivity of peroxidase compound II with phenols and anilines using the Marcus equation.
|
| |
Biol Chem,
386,
351-360.
|
 |
|
|
|
|
 |
M.Shintaku,
K.Matsuura,
S.Yoshioka,
S.Takahashi,
K.Ishimori,
and
I.Morishima
(2005).
Absence of a detectable intermediate in the compound I formation of horseradish peroxidase at ambient temperature.
|
| |
J Biol Chem,
280,
40934-40938.
|
 |
|
|
|
|
 |
O.Carugo,
and
K.Djinovi─ç Carugo
(2005).
When X-rays modify the protein structure: radiation damage at work.
|
| |
Trends Biochem Sci,
30,
213-219.
|
 |
|
|
|
|
 |
R.Silaghi-Dumitrescu,
and
C.E.Cooper
(2005).
Transient species involved in catalytic dioxygen/peroxide activation by hemoproteins: possible involvement of protonated Compound I species.
|
| |
Dalton Trans,
(),
3477-3482.
|
 |
|
|
|
|
 |
X.Carpena,
B.Wiseman,
T.Deemagarn,
R.Singh,
J.Switala,
A.Ivancich,
I.Fita,
and
P.C.Loewen
(2005).
A molecular switch and electronic circuit modulate catalase activity in catalase-peroxidases.
|
| |
EMBO Rep,
6,
1156-1162.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
Y.K.Choe,
and
S.Nagase
(2005).
Effect of the axial cysteine ligand on the electronic structure and reactivity of high-valent iron(IV) oxo-porphyrins (Compound I): a theoretical study.
|
| |
J Comput Chem,
26,
1600-1611.
|
 |
|
|
|
|
 |
J.M.Dias,
T.Alves,
C.Bonifácio,
A.S.Pereira,
J.Trincão,
D.Bourgeois,
I.Moura,
and
M.J.Romão
(2004).
Structural basis for the mechanism of Ca(2+) activation of the di-heme cytochrome c peroxidase from Pseudomonas nautica 617.
|
| |
Structure,
12,
961-973.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.Wang,
and
S.E.Ealick
(2004).
Observation of time-resolved structural changes by linear interpolation of highly redundant X-ray diffraction data.
|
| |
Acta Crystallogr D Biol Crystallogr,
60,
1579-1585.
|
 |
|
|
|
|
 |
J.Wuerges,
J.W.Lee,
Y.I.Yim,
H.S.Yim,
S.O.Kang,
and
K.Djinovic Carugo
(2004).
Crystal structure of nickel-containing superoxide dismutase reveals another type of active site.
|
| |
Proc Natl Acad Sci U S A,
101,
8569-8574.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.Nilsson,
H.P.Hersleth,
T.H.Rod,
K.K.Andersson,
and
U.Ryde
(2004).
The protonation status of compound II in myoglobin, studied by a combination of experimental data and quantum chemical calculations: quantum refinement.
|
| |
Biophys J,
87,
3437-3447.
|
 |
|
|
|
|
 |
M.A.Gilabert,
L.G.Fenoll,
F.García-Molina,
P.A.García-Ruiz,
J.Tudela,
F.García-Cánovas,
and
J.N.Rodríguez-López
(2004).
Stereospecificity of horseradish peroxidase.
|
| |
Biol Chem,
385,
1177-1184.
|
 |
|
|
|
|
 |
M.Schiltz,
P.Dumas,
E.Ennifar,
C.Flensburg,
W.Paciorek,
C.Vonrhein,
and
G.Bricogne
(2004).
Phasing in the presence of severe site-specific radiation damage through dose-dependent modelling of heavy atoms.
|
| |
Acta Crystallogr D Biol Crystallogr,
60,
1024-1031.
|
 |
|
|
|
|
 |
M.T.Green,
J.H.Dawson,
and
H.B.Gray
(2004).
Oxoiron(IV) in chloroperoxidase compound II is basic: implications for P450 chemistry.
|
| |
Science,
304,
1653-1656.
|
 |
|
|
|
|
 |
M.Unno,
T.Matsui,
G.C.Chu,
M.Couture,
T.Yoshida,
D.L.Rousseau,
J.S.Olson,
and
M.Ikeda-Saito
(2004).
Crystal structure of the dioxygen-bound heme oxygenase from Corynebacterium diphtheriae: implications for heme oxygenase function.
|
| |
J Biol Chem,
279,
21055-21061.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.Kort,
K.J.Hellingwerf,
and
R.B.Ravelli
(2004).
Initial events in the photocycle of photoactive yellow protein.
|
| |
J Biol Chem,
279,
26417-26424.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
V.Adam,
A.Royant,
V.Nivière,
F.P.Molina-Heredia,
and
D.Bourgeois
(2004).
Structure of superoxide reductase bound to ferrocyanide and active site expansion upon X-ray-induced photo-reduction.
|
| |
Structure,
12,
1729-1740.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
I.Bento,
V.H.Teixeira,
A.M.Baptista,
C.M.Soares,
P.M.Matias,
and
M.A.Carrondo
(2003).
Redox-Bohr and other cooperativity effects in the nine-heme cytochrome C from Desulfovibrio desulfuricans ATCC 27774: crystallographic and modeling studies.
|
| |
J Biol Chem,
278,
36455-36469.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.Diederichs,
S.McSweeney,
and
R.B.Ravelli
(2003).
Zero-dose extrapolation as part of macromolecular synchrotron data reduction.
|
| |
Acta Crystallogr D Biol Crystallogr,
59,
903-909.
|
 |
|
|
|
|
 |
K.H.Sharp,
M.Mewies,
P.C.Moody,
and
E.L.Raven
(2003).
Crystal structure of the ascorbate peroxidase-ascorbate complex.
|
| |
Nat Struct Biol,
10,
303-307.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Tanaka,
K.Matsuura,
S.Yoshioka,
S.Takahashi,
K.Ishimori,
H.Hori,
and
I.Morishima
(2003).
Activation of hydrogen peroxide in horseradish peroxidase occurs within approximately 200 micro s observed by a new freeze-quench device.
|
| |
Biophys J,
84,
1998-2004.
|
 |
|
|
|
|
 |
R.Fedorov,
I.Schlichting,
E.Hartmann,
T.Domratcheva,
M.Fuhrmann,
and
P.Hegemann
(2003).
Crystal structures and molecular mechanism of a light-induced signaling switch: The Phot-LOV1 domain from Chlamydomonas reinhardtii.
|
| |
Biophys J,
84,
2474-2482.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.R.Gabdoulline,
U.Kummer,
L.F.Olsen,
and
R.C.Wade
(2003).
Concerted simulations reveal how peroxidase compound III formation results in cellular oscillations.
|
| |
Biophys J,
85,
1421-1428.
|
 |
|
|
|
|
 |
I.G.Denisov,
T.M.Makris,
and
S.G.Sligar
(2002).
Formation and decay of hydroperoxo-ferric heme complex in horseradish peroxidase studied by cryoradiolysis.
|
| |
J Biol Chem,
277,
42706-42710.
|
 |
|
|
|
|
 |
K.Meno,
S.Jennings,
A.T.Smith,
A.Henriksen,
and
M.Gajhede
(2002).
Structural analysis of the two horseradish peroxidase catalytic residue variants H42E and R38S/H42E: implications for the catalytic cycle.
|
| |
Acta Crystallogr D Biol Crystallogr,
58,
1803-1812.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

