 |
PDBsum entry 2bhx

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.6.1.52
- phosphoserine transaminase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
O-phospho-L-serine + 2-oxoglutarate = 3-phosphooxypyruvate + L-glutamate
|
|
2.
|
4-(phosphooxy)-L-threonine + 2-oxoglutarate = (R)-3-hydroxy-2-oxo-4- phosphooxybutanoate + L-glutamate
|
|
 |
 |
 |
 |
 |
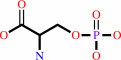 O-phospho-L-serine
O-phospho-L-serine
|
+
|
 2-oxoglutarate
2-oxoglutarate
|
=
|
3-phosphooxypyruvate
|
+
|
 L-glutamate
L-glutamate
|
|
 |
 |
 |
 |
 |
4-(phosphooxy)-L-threonine
|
+
|
 2-oxoglutarate
2-oxoglutarate
|
=
|
(R)-3-hydroxy-2-oxo-4- phosphooxybutanoate
|
+
|
 L-glutamate
L-glutamate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
Pyridoxal 5'-phosphate
Bound ligand (Het Group name =
PLP)
matches with 93.75% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Protein Sci
14:1498-1507
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Strain relief at the active site of phosphoserine aminotransferase induced by radiation damage.
|
|
A.P.Dubnovitsky,
R.B.Ravelli,
A.N.Popov,
A.C.Papageorgiou.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The X-ray susceptibility of the lysine-pyridoxal-5'-phosphate Schiff base in
Bacillus alcalophilus phosphoserine aminotransferase has been investigated using
crystallographic data collected at 100 K to 1.3 A resolution, complemented by
on-line spectroscopic studies. X-rays induce deprotonation of the internal
aldimine, changes in the Schiff base conformation, displacement of the cofactor
molecule, and disruption of the Schiff base linkage between
pyridoxal-5'-phosphate and the Lys residue. Analysis of the
"undamaged" structure reveals a significant chemical strain on the
internal aldimine bond that leads to a pronounced geometrical distortion of the
cofactor. However, upon crystal exposure to the X-rays, the strain and
distortion are relaxed and eventually diminished when the total absorbed dose
has exceeded 4.7 x 10(6) Ggamma. Our data provide new insights into the
enzymatic activation of pyridoxal-5'-phosphate and suggest that special care
should be taken while using macromolecular crystallography to study details in
strained active sites.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Figure 3. X-ray-induced structural changes in the active
site of BALC PSAT. Sigma A-weighted difference Fourier maps
(wF[A]-wF[n]) between the first data set, A, and the successive
data sets, B-G, respectively, are shown. Superimposed atomic
coordinates from the model A are presented. The green density
represents maps contoured at 6.5  , and the
blue density represents maps contoured at -6.0 , and the
blue density represents maps contoured at -6.0  . The figure
was produced using BOBSCRIPT (Esnouf 1997) and Raster 3D
(Merritt and Murphy 1994). (A) wF[A]-wF[B] map. (B) wF[A]-wF[C]
map. (C) wF[A]-wF[D] map. (D) wF[A]-wF[E] map. (E) wF[A]-wF[F]
map. (F) wF[A]-wF[G] map. . The figure
was produced using BOBSCRIPT (Esnouf 1997) and Raster 3D
(Merritt and Murphy 1994). (A) wF[A]-wF[B] map. (B) wF[A]-wF[C]
map. (C) wF[A]-wF[D] map. (D) wF[A]-wF[E] map. (E) wF[A]-wF[F]
map. (F) wF[A]-wF[G] map.
|
 |
Figure 6.
Figure 6. Distortion of pyridoxal-5'-phosphate in the
active site of BALC PSAT. Atomic coordinates of PLP and Lys196
side chain from the model H are presented in black. In gray, the
superimposed planar conformation of the PLP molecule from the
atomic resolution structure of BALC PSAT is shown (PDB accession
code 1W23). The figure was produced using MOLSCRIPT (Kraulis
1991) and Raster 3D (Merritt and Murphy 1994).
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the Protein Society:
Protein Sci
(2005,
14,
1498-1507)
copyright 2005.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
M.Schmidt,
V.Šrajer,
N.Purwar,
and
S.Tripathi
(2012).
The kinetic dose limit in room-temperature time-resolved macromolecular crystallography.
|
| |
J Synchrotron Radiat,
19,
264-273.
|
 |
|
|
|
|
 |
D.H.Juers,
and
M.Weik
(2011).
Similarities and differences in radiation damage at 100 K versus 160 K in a crystal of thermolysin.
|
| |
J Synchrotron Radiat,
18,
329-337.
|
 |
|
|
|
|
 |
E.F.Garman
(2010).
Radiation damage in macromolecular crystallography: what is it and why should we care?
|
| |
Acta Crystallogr D Biol Crystallogr,
66,
339-351.
|
 |
|
|
|
|
 |
G.P.Bourenkov,
and
A.N.Popov
(2010).
Optimization of data collection taking radiation damage into account.
|
| |
Acta Crystallogr D Biol Crystallogr,
66,
409-419.
|
 |
|
|
|
|
 |
M.Weik,
and
J.P.Colletier
(2010).
Temperature-dependent macromolecular X-ray crystallography.
|
| |
Acta Crystallogr D Biol Crystallogr,
66,
437-446.
|
 |
|
|
|
|
 |
P.Carpentier,
A.Royant,
M.Weik,
and
D.Bourgeois
(2010).
Raman-assisted crystallography suggests a mechanism of X-ray-induced disulfide radical formation and reparation.
|
| |
Structure,
18,
1410-1419.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.Petrova,
S.Ginell,
A.Mitschler,
Y.Kim,
V.Y.Lunin,
G.Joachimiak,
A.Cousido-Siah,
I.Hazemann,
A.Podjarny,
K.Lazarski,
and
A.Joachimiak
(2010).
X-ray-induced deterioration of disulfide bridges at atomic resolution.
|
| |
Acta Crystallogr D Biol Crystallogr,
66,
1075-1091.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
V.Mishra,
V.Ali,
T.Nozaki,
and
V.Bhakuni
(2010).
Entamoeba histolytica Phosphoserine aminotransferase (EhPSAT): insights into the structure-function relationship.
|
| |
BMC Res Notes,
3,
52.
|
 |
|
|
|
|
 |
B.Sjöblom,
M.Polentarutti,
and
K.Djinovic-Carugo
(2009).
Structural study of X-ray induced activation of carbonic anhydrase.
|
| |
Proc Natl Acad Sci U S A,
106,
10609-10613.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.M.Holton
(2009).
A beginner's guide to radiation damage.
|
| |
J Synchrotron Radiat,
16,
133-142.
|
 |
|
|
|
|
 |
J.McGeehan,
R.B.Ravelli,
J.W.Murray,
R.L.Owen,
F.Cipriani,
S.McSweeney,
M.Weik,
and
E.F.Garman
(2009).
Colouring cryo-cooled crystals: online microspectrophotometry.
|
| |
J Synchrotron Radiat,
16,
163-172.
|
 |
|
|
|
|
 |
R.L.Owen,
A.R.Pearson,
A.Meents,
P.Boehler,
V.Thominet,
and
C.Schulze-Briese
(2009).
A new on-axis multimode spectrometer for the macromolecular crystallography beamlines of the Swiss Light Source.
|
| |
J Synchrotron Radiat,
16,
173-182.
|
 |
|
|
|
|
 |
J.P.Colletier,
D.Bourgeois,
B.Sanson,
D.Fournier,
J.L.Sussman,
I.Silman,
and
M.Weik
(2008).
Shoot-and-Trap: use of specific x-ray damage to study structural protein dynamics by temperature-controlled cryo-crystallography.
|
| |
Proc Natl Acad Sci U S A,
105,
11742-11747.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.M.Holton
(2007).
XANES measurements of the rate of radiation damage to selenomethionine side chains.
|
| |
J Synchrotron Radiat,
14,
51-72.
|
 |
|
|
|
|
 |
T.De la Mora-Rey,
and
C.M.Wilmot
(2007).
Synergy within structural biology of single crystal optical spectroscopy and X-ray crystallography.
|
| |
Curr Opin Struct Biol,
17,
580-586.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

