 |
PDBsum entry 1asm

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Aminotransferase
|
PDB id
|
|

|

|
1asm
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.6.1.1
- aspartate transaminase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
L-aspartate + 2-oxoglutarate = oxaloacetate + L-glutamate
|
 |
 |
 |
 |
 |
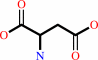 L-aspartate
L-aspartate
|
+
|
2-oxoglutarate
Bound ligand (Het Group name = )
matches with 88.89% similarity
|
=
|
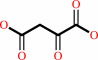 oxaloacetate
oxaloacetate
|
+
|
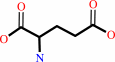 L-glutamate
L-glutamate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
Pyridoxal 5'-phosphate
Bound ligand (Het Group name =
PLP)
matches with 93.75% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
J Mol Biol
239:285-305
(1994)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structures of Escherichia coli aspartate aminotransferase in two conformations. Comparison of an unliganded open and two liganded closed forms.
|
|
J.Jäger,
M.Moser,
U.Sauder,
J.N.Jansonius.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Three crystal structures of wild type E. coli aspartate aminotransferase
(E.C.2.6.1.1) in space group P2(1) have been determined at resolution limits
between 2.6 and 2.35 A. The unliganded enzyme and its complexes with the
substrate analogues maleate and 2-methylaspartate resulted in different
conformations. The unit cell parameters of the unliganded and the inhibited
enzyme are a = 87.2, b = 79.9, c = 89.8 A and beta = 119.1 degrees, and a =
85.4, b = 79.8, c = 89.5 A and beta = 118.6 degrees, respectively. The
crystallographic symmetry is pseudo-C222(1). The liganded enzyme structures were
solved by difference Fourier techniques from that of a Val39-->Leu mutant
partially refined to an R-factor of 0.22 at 2.85 A. They have a "closed"
conformation like the chicken mAATase:maleate complex. The models were refined
to R-factors of 0.19 (maleate complex) and 0.18 (2-methylaspartate complex) by
molecular dynamics and restrained least squares methods. The unliganded crystal
form was solved by molecular replacement and refined to an R-factor of 0.19 at
2.5 A resolution. The structure is in a "half-open" conformation, with the small
domain rotated about 6 degrees from the closed conformation. The cofactor
pyridoxal phosphate has a more relaxed conformation than in mAATase. Both
maleate and 2-methylaspartate are hydrogen-bonded to the active site as in
mAATase. The C alpha-CH3 bond of 2-methylaspartate is oriented at right angles
to the cofactor pyridine ring, the most productive orientation for
alpha-deprotonation of the substrate L-aspartate. Comparisons with earlier
determined eAATase structures in space group C222(1) revealed differences that
can probably be attributed to the somewhat lower resolution of the orthorhombic
structures and/or mutations in the eAATases used in those studies. The present
P2(1) structures confirm the justification of extrapolating properties of active
site point mutants to the vertebrate isozymes. They will serve as reference in
the interpretation of the properties of further site-directed mutants in
continued studies of structure-function relationships of this enzyme.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
H.J.Wu,
Y.Yang,
S.Wang,
J.Q.Qiao,
Y.F.Xia,
Y.Wang,
W.D.Wang,
S.F.Gao,
J.Liu,
P.Q.Xue,
and
X.W.Gao
(2011).
Cloning, expression and characterization of a new aspartate aminotransferase from Bacillus subtilis B3.
|
| |
FEBS J,
278,
1345-1357.
|
 |
|
|
|
|
 |
Q.Han,
H.Robinson,
T.Cai,
D.A.Tagle,
and
J.Li
(2011).
Biochemical and structural characterization of mouse mitochondrial aspartate aminotransferase, a newly identified kynurenine aminotransferase-IV.
|
| |
Biosci Rep,
31,
323-332.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
Q.Han,
T.Cai,
D.A.Tagle,
and
J.Li
(2010).
Structure, expression, and function of kynurenine aminotransferases in human and rodent brains.
|
| |
Cell Mol Life Sci,
67,
353-368.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
T.Lendrihas,
G.A.Hunter,
and
G.C.Ferreira
(2010).
Serine 254 enhances an induced fit mechanism in murine 5-aminolevulinate synthase.
|
| |
J Biol Chem,
285,
3351-3359.
|
 |
|
|
|
|
 |
M.Goto,
T.Yamauchi,
N.Kamiya,
I.Miyahara,
T.Yoshimura,
H.Mihara,
T.Kurihara,
K.Hirotsu,
and
N.Esaki
(2009).
Crystal structure of a homolog of mammalian serine racemase from Schizosaccharomyces pombe.
|
| |
J Biol Chem,
284,
25944-25952.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.Tomita,
T.Miyagawa,
T.Miyazaki,
S.Fushinobu,
T.Kuzuyama,
and
M.Nishiyama
(2009).
Mechanism for multiple-substrates recognition of alpha-aminoadipate aminotransferase from Thermus thermophilus.
|
| |
Proteins,
75,
348-359.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.M.Thornburg,
K.K.Nelson,
B.F.Clem,
A.N.Lane,
S.Arumugam,
A.Simmons,
J.W.Eaton,
S.Telang,
and
J.Chesney
(2008).
Targeting aspartate aminotransferase in breast cancer.
|
| |
Breast Cancer Res,
10,
R84.
|
 |
|
|
|
|
 |
Q.Han,
H.Robinson,
and
J.Li
(2008).
Crystal Structure of Human Kynurenine Aminotransferase II.
|
| |
J Biol Chem,
283,
3567-3573.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
Q.Han,
T.Cai,
D.A.Tagle,
H.Robinson,
and
J.Li
(2008).
Substrate specificity and structure of human aminoadipate aminotransferase/kynurenine aminotransferase II.
|
| |
Biosci Rep,
28,
205-215.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
Q.Han,
Y.G.Gao,
H.Robinson,
and
J.Li
(2008).
Structural insight into the mechanism of substrate specificity of aedes kynurenine aminotransferase.
|
| |
Biochemistry,
47,
1622-1630.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.Z.Liao,
W.J.Ding,
J.G.Yu,
W.H.Fang,
and
R.Z.Liu
(2008).
Theoretical studies on pyridoxal 5'-phosphate-dependent transamination of alpha-amino acids.
|
| |
J Comput Chem,
29,
1919-1929.
|
 |
|
|
|
|
 |
S.Lima,
R.Khristoforov,
C.Momany,
and
R.S.Phillips
(2007).
Crystal structure of Homo sapiens kynureninase.
|
| |
Biochemistry,
46,
2735-2744.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
N.H.Yennawar,
M.M.Islam,
M.Conway,
R.Wallin,
and
S.M.Hutson
(2006).
Human mitochondrial branched chain aminotransferase isozyme: structural role of the CXXC center in catalysis.
|
| |
J Biol Chem,
281,
39660-39671.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.Hirotsu,
M.Goto,
A.Okamoto,
and
I.Miyahara
(2005).
Dual substrate recognition of aminotransferases.
|
| |
Chem Rec,
5,
160-172.
|
 |
|
|
|
|
 |
M.Goto,
R.Omi,
I.Miyahara,
A.Hosono,
H.Mizuguchi,
H.Hayashi,
H.Kagamiyama,
and
K.Hirotsu
(2004).
Crystal structures of glutamine:phenylpyruvate aminotransferase from Thermus thermophilus HB8: induced fit and substrate recognition.
|
| |
J Biol Chem,
279,
16518-16525.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.C.Rothman,
M.Voorhies,
and
J.F.Kirsch
(2004).
Directed evolution relieves product inhibition and confers in vivo function to a rationally designed tyrosine aminotransferase.
|
| |
Protein Sci,
13,
763-772.
|
 |
|
|
|
|
 |
Y.Katsura,
M.Shirouzu,
H.Yamaguchi,
R.Ishitani,
O.Nureki,
S.Kuramitsu,
H.Hayashi,
and
S.Yokoyama
(2004).
Crystal structure of a putative aspartate aminotransferase belonging to subgroup IV.
|
| |
Proteins,
55,
487-492.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
H.Hayashi,
H.Mizuguchi,
I.Miyahara,
Y.Nakajima,
K.Hirotsu,
and
H.Kagamiyama
(2003).
Conformational change in aspartate aminotransferase on substrate binding induces strain in the catalytic group and enhances catalysis.
|
| |
J Biol Chem,
278,
9481-9488.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
H.Kim,
K.Ikegami,
M.Nakaoka,
M.Yagi,
H.Shibata,
and
Y.Sawa
(2003).
Characterization of aspartate aminotransferase from the cyanobacterium Phormidium lapideum.
|
| |
Biosci Biotechnol Biochem,
67,
490-498.
|
 |
|
|
|
|
 |
H.Kim,
M.Nakaoka,
M.Yagi,
H.Ashida,
K.Hamada,
H.Shibata,
and
Y.Sawa
(2003).
Cloning, structural analysis and expression of the gene encoding aspartate aminotransferase from the thermophilic cyanobacterium Phormidium lapideum.
|
| |
J Biosci Bioeng,
95,
421-424.
|
 |
|
|
|
|
 |
J.K.Yang,
C.Chang,
S.J.Cho,
J.Y.Lee,
Y.G.Yu,
S.H.Eom,
and
S.W.Suh
(2003).
Crystallization and preliminary X-ray analysis of the Mj0684 gene product, a putative aspartate aminotransferase, from Methanococcus jannaschii.
|
| |
Acta Crystallogr D Biol Crystallogr,
59,
563-565.
|
 |
|
|
|
|
 |
R.Omi,
M.Goto,
I.Miyahara,
H.Mizuguchi,
H.Hayashi,
H.Kagamiyama,
and
K.Hirotsu
(2003).
Crystal structures of threonine synthase from Thermus thermophilus HB8: conformational change, substrate recognition, and mechanism.
|
| |
J Biol Chem,
278,
46035-46045.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
E.Deu,
K.A.Koch,
and
J.F.Kirsch
(2002).
The role of the conserved Lys68*:Glu265 intersubunit salt bridge in aspartate aminotransferase kinetics: multiple forced covariant amino acid substitutions in natural variants.
|
| |
Protein Sci,
11,
1062-1073.
|
 |
|
|
|
|
 |
J.Zhang,
and
G.C.Ferreira
(2002).
Transient state kinetic investigation of 5-aminolevulinate synthase reaction mechanism.
|
| |
J Biol Chem,
277,
44660-44669.
|
 |
|
|
|
|
 |
L.Birolo,
F.Dal Piaz,
P.Pucci,
and
G.Marino
(2002).
Structural characterization of the M* partly folded intermediate of wild type and P138A aspartate aminotransferase from Escherichia coli.
|
| |
J Biol Chem,
277,
17428-17437.
|
 |
|
|
|
|
 |
O.Hur,
D.Niks,
P.Casino,
and
M.F.Dunn
(2002).
Proton transfers in the beta-reaction catalyzed by tryptophan synthase.
|
| |
Biochemistry,
41,
9991.
|
 |
|
|
|
|
 |
V.Trivedi,
A.Gupta,
V.R.Jala,
P.Saravanan,
G.S.Rao,
N.A.Rao,
H.S.Savithri,
and
H.S.Subramanya
(2002).
Crystal structure of binary and ternary complexes of serine hydroxymethyltransferase from Bacillus stearothermophilus: insights into the catalytic mechanism.
|
| |
J Biol Chem,
277,
17161-17169.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Matharu,
H.Hayashi,
H.Kagamiyama,
B.Maras,
and
R.A.John
(2001).
Contributions of the substrate-binding arginine residues to maleate-induced closure of the active site of Escherichia coli aspartate aminotransferase.
|
| |
Eur J Biochem,
268,
1640-1645.
|
 |
|
|
|
|
 |
H.Kagamiyama,
and
H.Hayashi
(2001).
Release of enzyme strain during catalysis reduces the activation energy barrier.
|
| |
Chem Rec,
1,
385-394.
|
 |
|
|
|
|
 |
K.Haruyama,
T.Nakai,
I.Miyahara,
K.Hirotsu,
H.Mizuguchi,
H.Hayashi,
and
H.Kagamiyama
(2001).
Structures of Escherichia coli histidinol-phosphate aminotransferase and its complexes with histidinol-phosphate and N-(5'-phosphopyridoxyl)-L-glutamate: double substrate recognition of the enzyme.
|
| |
Biochemistry,
40,
4633-4644.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
N.Yennawar,
J.Dunbar,
M.Conway,
S.Hutson,
and
G.Farber
(2001).
The structure of human mitochondrial branched-chain aminotransferase.
|
| |
Acta Crystallogr D Biol Crystallogr,
57,
506-515.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.Contestabile,
A.Paiardini,
S.Pascarella,
M.L.di Salvo,
S.D'Aguanno,
and
F.Bossa
(2001).
l-Threonine aldolase, serine hydroxymethyltransferase and fungal alanine racemase. A subgroup of strictly related enzymes specialized for different functions.
|
| |
Eur J Biochem,
268,
6508-6525.
|
 |
|
|
|
|
 |
G.Schneider,
H.Käck,
and
Y.Lindqvist
(2000).
The manifold of vitamin B6 dependent enzymes.
|
| |
Structure,
8,
R1-R6.
|
 |
|
|
|
|
 |
J.Ishijima,
T.Nakai,
S.Kawaguchi,
K.Hirotsu,
and
S.Kuramitsu
(2000).
Free energy requirement for domain movement of an enzyme.
|
| |
J Biol Chem,
275,
18939-18945.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.Birolo,
M.L.Tutino,
B.Fontanella,
C.Gerday,
K.Mainolfi,
S.Pascarella,
G.Sannia,
F.Vinci,
and
G.Marino
(2000).
Aspartate aminotransferase from the Antarctic bacterium Pseudoalteromonas haloplanktis TAC 125. Cloning, expression, properties, and molecular modelling.
|
| |
Eur J Biochem,
267,
2790-2802.
|
 |
|
|
|
|
 |
B.Mouratou,
P.Kasper,
H.Gehring,
and
P.Christen
(1999).
Conversion of tyrosine phenol-lyase to dicarboxylic amino acid beta-lyase, an enzyme not found in nature.
|
| |
J Biol Chem,
274,
1320-1325.
|
 |
|
|
|
|
 |
G.A.Hunter,
and
G.C.Ferreira
(1999).
Pre-steady-state reaction of 5-aminolevulinate synthase. Evidence for a rate-determining product release.
|
| |
J Biol Chem,
274,
12222-12228.
|
 |
|
|
|
|
 |
K.A.Denessiouk,
A.I.Denesyuk,
J.V.Lehtonen,
T.Korpela,
and
M.S.Johnson
(1999).
Common structural elements in the architecture of the cofactor-binding domains in unrelated families of pyridoxal phosphate-dependent enzymes.
|
| |
Proteins,
35,
250-261.
|
 |
|
|
|
|
 |
R.Graber,
P.Kasper,
V.N.Malashkevich,
P.Strop,
H.Gehring,
J.N.Jansonius,
and
P.Christen
(1999).
Conversion of aspartate aminotransferase into an L-aspartate beta-decarboxylase by a triple active-site mutation.
|
| |
J Biol Chem,
274,
31203-31208.
|
 |
|
|
|
|
 |
S.Oue,
A.Okamoto,
T.Yano,
and
H.Kagamiyama
(1999).
Redesigning the substrate specificity of an enzyme by cumulative effects of the mutations of non-active site residues.
|
| |
J Biol Chem,
274,
2344-2349.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Azzariti,
R.A.Vacca,
S.Giannattasio,
R.S.Merafina,
E.Marra,
and
S.Doonan
(1998).
Kinetic properties and thermal stabilities of mutant forms of mitochondrial aspartate aminotransferase.
|
| |
Biochim Biophys Acta,
1386,
29-38.
|
 |
|
|
|
|
 |
C.J.Jeffery,
T.Barry,
S.Doonan,
G.A.Petsko,
and
D.Ringe
(1998).
Crystal structure of Saccharomyces cerevisiae cytosolic aspartate aminotransferase.
|
| |
Protein Sci,
7,
1380-1387.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
D.Peisach,
D.M.Chipman,
P.W.Van Ophem,
J.M.Manning,
and
D.Ringe
(1998).
Crystallographic study of steps along the reaction pathway of D-amino acid aminotransferase.
|
| |
Biochemistry,
37,
4958-4967.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
D.T.Gallagher,
G.L.Gilliland,
G.Xiao,
J.Zondlo,
K.E.Fisher,
D.Chinchilla,
and
E.Eisenstein
(1998).
Structure and control of pyridoxal phosphate dependent allosteric threonine deaminase.
|
| |
Structure,
6,
465-475.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
G.F.Stamper,
A.A.Morollo,
D.Ringe,
and
C.G.Stamper
(1998).
Reaction of alanine racemase with 1-aminoethylphosphonic acid forms a stable external aldimine.
|
| |
Biochemistry,
37,
10438-10445.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
Y.Nobe,
S.Kawaguchi,
H.Ura,
T.Nakai,
K.Hirotsu,
R.Kato,
and
S.Kuramitsu
(1998).
The novel substrate recognition mechanism utilized by aspartate aminotransferase of the extreme thermophile Thermus thermophilus HB8.
|
| |
J Biol Chem,
273,
29554-29564.
|
 |
|
|
|
|
 |
A.Artigues,
A.Iriarte,
and
M.Martinez-Carrion
(1997).
Refolding intermediates of acid-unfolded mitochondrial aspartate aminotransferase bind to hsp70.
|
| |
J Biol Chem,
272,
16852-16861.
|
 |
|
|
|
|
 |
D.M.van Aalten,
D.A.Conn,
B.L.de Groot,
H.J.Berendsen,
J.B.Findlay,
and
A.Amadei
(1997).
Protein dynamics derived from clusters of crystal structures.
|
| |
Biophys J,
73,
2891-2896.
|
 |
|
|
|
|
 |
E.T.Mollova,
D.E.Metzler,
A.Kintanar,
H.Kagamiyama,
H.Hayashi,
K.Hirotsu,
and
I.Miyahara
(1997).
Use of 1H-15N heteronuclear multiple-quantum coherence NMR spectroscopy to study the active site of aspartate aminotransferase.
|
| |
Biochemistry,
36,
615-625.
|
 |
|
|
|
|
 |
M.Bergdoll,
M.H.Remy,
C.Cagnon,
J.M.Masson,
and
P.Dumas
(1997).
Proline-dependent oligomerization with arm exchange.
|
| |
Structure,
5,
391-401.
|
 |
|
|
|
|
 |
R.A.Vacca,
S.Giannattasio,
R.Graber,
E.Sandmeier,
E.Marra,
and
P.Christen
(1997).
Active-site Arg --> Lys substitutions alter reaction and substrate specificity of aspartate aminotransferase.
|
| |
J Biol Chem,
272,
21932-21937.
|
 |
|
|
|
|
 |
J.M.Goldberg,
and
J.F.Kirsch
(1996).
The reaction catalyzed by Escherichia coli aspartate aminotransferase has multiple partially rate-determining steps, while that catalyzed by the Y225F mutant is dominated by ketimine hydrolysis.
|
| |
Biochemistry,
35,
5280-5291.
|
 |
|
|
|
|
 |
S.Vaccari,
S.Benci,
A.Peracchi,
and
A.Mozzarelli
(1996).
Time-resolved fluorescence of tryptophan synthase.
|
| |
Biophys Chem,
61,
9.
|
 |
|
|
|
|
 |
H.Chen,
P.Gollnick,
and
R.S.Phillips
(1995).
Site-directed mutagenesis of His343-->Ala in Citrobacter freundii tyrosine phenol-lyase. Effects on the kinetic mechanism and rate-determining step.
|
| |
Eur J Biochem,
229,
540-549.
|
 |
|
|
|
|
 |
J.J.Onuffer,
B.T.Ton,
I.Klement,
and
J.F.Kirsch
(1995).
The use of natural and unnatural amino acid substrates to define the substrate specificity differences of Escherichia coli aspartate and tyrosine aminotransferases.
|
| |
Protein Sci,
4,
1743-1749.
|
 |
|
|
|
|
 |
J.J.Onuffer,
and
J.F.Kirsch
(1995).
Redesign of the substrate specificity of Escherichia coli aspartate aminotransferase to that of Escherichia coli tyrosine aminotransferase by homology modeling and site-directed mutagenesis.
|
| |
Protein Sci,
4,
1750-1757.
|
 |
|
|
|
|
 |
L.Birolo,
E.Sandmeier,
P.Christen,
and
R.A.John
(1995).
The roles of Tyr70 and Tyr225 in aspartate aminotransferase assessed by analysing the effects of mutations on the multiple reactions of the substrate analogue serine o-sulphate.
|
| |
Eur J Biochem,
232,
859-864.
|
 |
|
|
|
|
 |
M.D.Toney,
S.Pascarella,
and
D.De Biase
(1995).
Active site model for gamma-aminobutyrate aminotransferase explains substrate specificity and inhibitor reactivities.
|
| |
Protein Sci,
4,
2366-2374.
|
 |
|
|
|
|
 |
R.A.Vacca,
P.Christen,
V.N.Malashkevich,
J.N.Jansonius,
and
E.Sandmeier
(1995).
Substitution of apolar residues in the active site of aspartate aminotransferase by histidine. Effects on reaction and substrate specificity.
|
| |
Eur J Biochem,
227,
481-487.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.Buchli,
D.Alberati-Giani,
P.Malherbe,
C.Köhler,
C.Broger,
and
A.M.Cesura
(1995).
Cloning and functional expression of a soluble form of kynurenine/alpha-aminoadipate aminotransferase from rat kidney.
|
| |
J Biol Chem,
270,
29330-29335.
|
 |
|
|
|
|
 |
R.Graber,
P.Kasper,
V.N.Malashkevich,
E.Sandmeier,
P.Berger,
H.Gehring,
J.N.Jansonius,
and
P.Christen
(1995).
Changing the reaction specificity of a pyridoxal-5'-phosphate-dependent enzyme.
|
| |
Eur J Biochem,
232,
686-690.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
V.N.Malashkevich,
J.J.Onuffer,
J.F.Kirsch,
and
J.N.Jansonius
(1995).
Alternating arginine-modulated substrate specificity in an engineered tyrosine aminotransferase.
|
| |
Nat Struct Biol,
2,
548-553.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
X.Wu,
B.Knudsen,
S.M.Feller,
J.Zheng,
A.Sali,
D.Cowburn,
H.Hanafusa,
and
J.Kuriyan
(1995).
Structural basis for the specific interaction of lysine-containing proline-rich peptides with the N-terminal SH3 domain of c-Crk.
|
| |
Structure,
3,
215-226.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

