 |
PDBsum entry 1sox

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1sox
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.8.3.1
- sulfite oxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
sulfite + O2 + H2O = sulfate + H2O2
|
 |
 |
 |
 |
 |
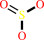 sulfite
sulfite
|
+
|
 O2
O2
|
+
|
H2O
|
=
|
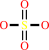 sulfate
sulfate
|
+
|
H2O2
Bound ligand (Het Group name = )
corresponds exactly
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Heme; Mo cation
|
 |
 |
 |
 |
 |
Heme
Bound ligand (Het Group name =
HEM)
matches with 95.45% similarity
|
Mo cation
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Cell
91:973-983
(1997)
|
|
PubMed id:
|
|
|
|

|
| |
|
Molecular basis of sulfite oxidase deficiency from the structure of sulfite oxidase.
|
|
C.Kisker,
H.Schindelin,
A.Pacheco,
W.A.Wehbi,
R.M.Garrett,
K.V.Rajagopalan,
J.H.Enemark,
D.C.Rees.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The molybdenum-containing enzyme sulfite oxidase catalyzes the conversion of
sulfite to sulfate, the terminal step in the oxidative degradation of cysteine
and methionine. Deficiency of this enzyme in humans usually leads to major
neurological abnormalities and early death. The crystal structure of chicken
liver sulfite oxidase at 1.9 A resolution reveals that each monomer of the
dimeric enzyme consists of three domains. At the active site, the Mo is
penta-coordinated by three sulfur ligands, one oxo group, and one water/hydroxo.
A sulfate molecule adjacent to the Mo identifies the substrate binding pocket.
Four variants associated with sulfite oxidase deficiency have been identified:
two mutations are near the sulfate binding site, while the other mutations occur
within the domain mediating dimerization.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Figure 2. Structure of Sulfite Oxidase(A) The monomer with
the N-terminal domain drawn in red, the Mo-co domain in yellow,
and the C-terminal domain in green. The N and the C termini are
labeled with N and C, respectively. The dotted line between
domains I and II indicates the loop region, which is only weakly
defined in the electron density. The Mo-co and the heme are
shown in ball-and-stick representation with the Fe atom in
purple and the Mo atom in green. Figure 2 and Figure 3B have
been generated with the programs MOLSCRIPT ( [30]) and RASTER3D
( [1 and 34]).(B) Cα trace of the sulfite oxidase dimer. The
gray dotted lines connect the metal centers of the cofactors and
indicate the distances between the Mo and Fe within the monomer
and the Mo-Mo distance between the two monomers.(C) Interface
between the N-terminal and the Mo-co domain. Aliphatic residues
are shown in all-bonds representation whereas the heme and
residues involved in hydrogen bonding are shown in
ball-and-stick representation. Dotted lines indicate hydrogen
bonds.
|
 |
Figure 3.
Figure 3. The Active Site(A) Schematic representation of
all protein–cofactor interactions. No water-mediated hydrogen
bonds between the cofactor and the protein are observed.
Hydrogen bonds are indicated by dashed lines.(B) Stereo view of
the active site. All residues are shown in ball-and-stick
representation. Atoms which coordinate the Mo are connected to
the Mo (green) by a solid line, the view is approximately along
the bond between the Mo and the axial oxo ligand.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Cell Press:
Cell
(1997,
91,
973-983)
copyright 1997.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
P.V.Bernhardt
(2011).
Exploiting the versatility and selectivity of Mo enzymes with electrochemistry.
|
| |
Chem Commun (Camb),
47,
1663-1673.
|
 |
|
|
|
|
 |
R.Mayilmurugan,
B.N.Harum,
M.Volpe,
A.F.Sax,
M.Palaniandavar,
and
N.C.Mösch-Zanetti
(2011).
Mechanistic insight into the reactivity of oxotransferases by novel asymmetric dioxomolybdenum(VI) model complexes.
|
| |
Chemistry,
17,
704-713.
|
 |
|
|
|
|
 |
C.Drögemüller,
J.Tetens,
S.Sigurdsson,
A.Gentile,
S.Testoni,
K.Lindblad-Toh,
and
T.Leeb
(2010).
Identification of the bovine Arachnomelia mutation by massively parallel sequencing implicates sulfite oxidase (SUOX) in bone development.
|
| |
PLoS Genet,
6,
0.
|
 |
|
|
|
|
 |
J.McMaster,
and
V.S.Oganesyan
(2010).
Magnetic circular dichroism spectroscopy as a probe of the structures of the metal sites in metalloproteins.
|
| |
Curr Opin Struct Biol,
20,
615-622.
|
 |
|
|
|
|
 |
K.Johnson-Winters,
A.R.Nordstrom,
A.C.Davis,
G.Tollin,
and
J.H.Enemark
(2010).
Effects of large-scale amino acid substitution in the polypeptide tether connecting the heme and molybdenum domains on catalysis in human sulfite oxidase.
|
| |
Metallomics,
2,
766-770.
|
 |
|
|
|
|
 |
K.Johnson-Winters,
A.R.Nordstrom,
S.Emesh,
A.V.Astashkin,
A.Rajapakshe,
R.E.Berry,
G.Tollin,
and
J.H.Enemark
(2010).
Effects of interdomain tether length and flexibility on the kinetics of intramolecular electron transfer in human sulfite oxidase.
|
| |
Biochemistry,
49,
1290-1296.
|
 |
|
|
|
|
 |
T.D.Rapson,
A.V.Astashkin,
K.Johnson-Winters,
P.V.Bernhardt,
U.Kappler,
A.M.Raitsimring,
and
J.H.Enemark
(2010).
Pulsed EPR investigations of the Mo(V) centers of the R55Q and R55M variants of sulfite dehydrogenase from Starkeya novella.
|
| |
J Biol Inorg Chem,
15,
505-514.
|
 |
|
|
|
|
 |
A.V.Astashkin,
E.L.Klein,
D.Ganyushin,
K.Johnson-Winters,
F.Neese,
U.Kappler,
and
J.H.Enemark
(2009).
Exchangeable oxygens in the vicinity of the molybdenum center of the high-pH form of sulfite oxidase and sulfite dehydrogenase.
|
| |
Phys Chem Chem Phys,
11,
6733-6742.
|
 |
|
|
|
|
 |
E.L.Klein,
A.V.Astashkin,
D.Ganyushin,
C.Riplinger,
K.Johnson-Winters,
F.Neese,
and
J.H.Enemark
(2009).
Direct detection and characterization of chloride in the active site of the low-pH form of sulfite oxidase using electron spin echo envelope modulation spectroscopy, isotopic labeling, and density functional theory calculations.
|
| |
Inorg Chem,
48,
4743-4752.
|
 |
|
|
|
|
 |
G.Schwarz,
R.R.Mendel,
and
M.W.Ribbe
(2009).
Molybdenum cofactors, enzymes and pathways.
|
| |
Nature,
460,
839-847.
|
 |
|
|
|
|
 |
M.J.Romão
(2009).
Molybdenum and tungsten enzymes: a crystallographic and mechanistic overview.
|
| |
Dalton Trans,
(),
4053-4068.
|
 |
|
|
|
|
 |
R.R.Mendel
(2009).
Cell biology of molybdenum.
|
| |
Biofactors,
35,
429-434.
|
 |
|
|
|
|
 |
S.Bailey,
T.Rapson,
K.Johnson-Winters,
A.V.Astashkin,
J.H.Enemark,
and
U.Kappler
(2009).
Molecular basis for enzymatic sulfite oxidation: how three conserved active site residues shape enzyme activity.
|
| |
J Biol Chem,
284,
2053-2063.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Emesh,
T.D.Rapson,
A.Rajapakshe,
U.Kappler,
P.V.Bernhardt,
G.Tollin,
and
J.H.Enemark
(2009).
Intramolecular electron transfer in sulfite-oxidizing enzymes: elucidating the role of a conserved active site arginine.
|
| |
Biochemistry,
48,
2156-2163.
|
 |
|
|
|
|
 |
S.Groysman,
and
R.H.Holm
(2009).
Biomimetic chemistry of iron, nickel, molybdenum, and tungsten in sulfur-ligated protein sites.
|
| |
Biochemistry,
48,
2310-2320.
|
 |
|
|
|
|
 |
A.M.Raitsimring,
A.V.Astashkin,
C.Feng,
H.L.Wilson,
K.V.Rajagopalan,
and
J.H.Enemark
(2008).
Studies of the Mo(V) Center of the Y343F Mutant of Human Sulfite Oxidase by Variable Frequency Pulsed EPR Spectroscopy.
|
| |
Inorganica Chim Acta,
361,
941-946.
|
 |
|
|
|
|
 |
A.V.Astashkin,
K.Johnson-Winters,
E.L.Klein,
C.Feng,
H.L.Wilson,
K.V.Rajagopalan,
A.M.Raitsimring,
and
J.H.Enemark
(2008).
Structural studies of the molybdenum center of the pathogenic R160Q mutant of human sulfite oxidase by pulsed EPR spectroscopy and 17O and 33S labeling.
|
| |
J Am Chem Soc,
130,
8471-8480.
|
 |
|
|
|
|
 |
B.Smolinsky,
S.A.Eichler,
S.Buchmeier,
J.C.Meier,
and
G.Schwarz
(2008).
Splice-specific functions of gephyrin in molybdenum cofactor biosynthesis.
|
| |
J Biol Chem,
283,
17370-17379.
|
 |
|
|
|
|
 |
C.Nacitarhan,
V.Kucukatay,
G.Sadan,
O.H.Ozturk,
and
A.Agar
(2008).
Effects of sulphite supplementation on vascular responsiveness in sulphite oxidase-deficient rats.
|
| |
Clin Exp Pharmacol Physiol,
35,
268-272.
|
 |
|
|
|
|
 |
G.J.Workun,
K.Moquin,
R.A.Rothery,
and
J.H.Weiner
(2008).
Evolutionary persistence of the molybdopyranopterin-containing sulfite oxidase protein fold.
|
| |
Microbiol Mol Biol Rev,
72,
228.
|
 |
|
|
|
|
 |
J.H.Enemark,
A.V.Astashkin,
and
A.M.Raitsimring
(2008).
Structures and reaction pathways of the molybdenum centres of sulfite-oxidizing enzymes by pulsed EPR spectroscopy.
|
| |
Biochem Soc Trans,
36,
1129-1133.
|
 |
|
|
|
|
 |
R.S.Sengar,
J.J.Miller,
and
P.Basu
(2008).
Design, syntheses, and characterization of dioxo-molybdenum(VI) complexes with thiolate ligands: effects of intraligand NH...S hydrogen bonding.
|
| |
Dalton Trans,
(),
2569-2577.
|
 |
|
|
|
|
 |
A.M.Burroughs,
S.Balaji,
L.M.Iyer,
and
L.Aravind
(2007).
A novel superfamily containing the beta-grasp fold involved in binding diverse soluble ligands.
|
| |
Biol Direct,
2,
4.
|
 |
|
|
|
|
 |
A.M.Burroughs,
S.Balaji,
L.M.Iyer,
and
L.Aravind
(2007).
Small but versatile: the extraordinary functional and structural diversity of the beta-grasp fold.
|
| |
Biol Direct,
2,
18.
|
 |
|
|
|
|
 |
A.V.Astashkin,
K.Johnson-Winters,
E.L.Klein,
R.S.Byrne,
R.Hille,
A.M.Raitsimring,
and
J.H.Enemark
(2007).
Direct demonstration of the presence of coordinated sulfate in the reaction pathway of Arabidopsis thaliana sulfite oxidase using 33S labeling and ESEEM spectroscopy.
|
| |
J Am Chem Soc,
129,
14800-14810.
|
 |
|
|
|
|
 |
C.Feng,
G.Tollin,
and
J.H.Enemark
(2007).
Sulfite oxidizing enzymes.
|
| |
Biochim Biophys Acta,
1774,
527-539.
|
 |
|
|
|
|
 |
G.B.Seiffert,
G.M.Ullmann,
A.Messerschmidt,
B.Schink,
P.M.Kroneck,
and
O.Einsle
(2007).
Structure of the non-redox-active tungsten/[4Fe:4S] enzyme acetylene hydratase.
|
| |
Proc Natl Acad Sci U S A,
104,
3073-3077.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.Pal,
P.K.Chaudhury,
and
S.Sarkar
(2007).
Structure of the Michaelis complex and function of the catalytic center in the reductive half-reaction of computational and synthetic models of sulfite oxidase.
|
| |
Chem Asian J,
2,
956-964.
|
 |
|
|
|
|
 |
M.A.Cranswick,
A.Dawson,
J.J.Cooney,
N.E.Gruhn,
D.L.Lichtenberger,
and
J.H.Enemark
(2007).
Photoelectron spectroscopy and electronic structure calculations of d1 vanadocene compounds with chelated dithiolate ligands: implications for pyranopterin Mo/W enzymes.
|
| |
Inorg Chem,
46,
10639-10646.
|
 |
|
|
|
|
 |
R.Hänsch,
C.Lang,
H.Rennenberg,
and
R.R.Mendel
(2007).
Significance of plant sulfite oxidase.
|
| |
Plant Biol (Stuttg),
9,
589-595.
|
 |
|
|
|
|
 |
R.R.Mendel,
A.G.Smith,
A.Marquet,
and
M.J.Warren
(2007).
Metal and cofactor insertion.
|
| |
Nat Prod Rep,
24,
963-971.
|
 |
|
|
|
|
 |
A.Di Salle,
G.D'Errico,
F.La Cara,
R.Cannio,
and
M.Rossi
(2006).
A novel thermostable sulfite oxidase from Thermus thermophilus: characterization of the enzyme, gene cloning and expression in Escherichia coli.
|
| |
Extremophiles,
10,
587-598.
|
 |
|
|
|
|
 |
A.Llamas,
T.Otte,
G.Multhaup,
R.R.Mendel,
and
G.Schwarz
(2006).
The Mechanism of nucleotide-assisted molybdenum insertion into molybdopterin. A novel route toward metal cofactor assembly.
|
| |
J Biol Chem,
281,
18343-18350.
|
 |
|
|
|
|
 |
G.Schwarz,
and
R.R.Mendel
(2006).
Molybdenum cofactor biosynthesis and molybdenum enzymes.
|
| |
Annu Rev Plant Biol,
57,
623-647.
|
 |
|
|
|
|
 |
J.H.Enemark,
A.V.Astashkin,
and
A.M.Raitsimring
(2006).
Investigation of the coordination structures of the molybdenum(v) sites of sulfite oxidizing enzymes by pulsed EPR spectroscopy.
|
| |
Dalton Trans,
(),
3501-3514.
|
 |
|
|
|
|
 |
K.Fischer,
A.Llamas,
M.Tejada-Jimenez,
N.Schrader,
J.Kuper,
F.S.Ataya,
A.Galvan,
R.R.Mendel,
E.Fernandez,
and
G.Schwarz
(2006).
Function and structure of the molybdenum cofactor carrier protein from Chlamydomonas reinhardtii.
|
| |
J Biol Chem,
281,
30186-30194.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.Hänsch,
C.Lang,
E.Riebeseel,
R.Lindigkeit,
A.Gessler,
H.Rennenberg,
and
R.R.Mendel
(2006).
Plant sulfite oxidase as novel producer of H2O2: combination of enzyme catalysis with a subsequent non-enzymatic reaction step.
|
| |
J Biol Chem,
281,
6884-6888.
|
 |
|
|
|
|
 |
A.F.Peacock,
H.D.Batey,
C.Raendler,
A.C.Whitwood,
R.N.Perutz,
and
A.K.Duhme-Klair
(2005).
A metal-based lumophore tailored to sense biologically relevant oxometalates.
|
| |
Angew Chem Int Ed Engl,
44,
1712-1714.
|
 |
|
|
|
|
 |
A.J.Millar,
C.J.Doonan,
P.D.Smith,
V.N.Nemykin,
P.Basu,
and
C.G.Young
(2005).
Oxygen atom transfer in models for molybdenum enzymes: isolation and structural, spectroscopic, and computational studies of intermediates in oxygen atom transfer from molybdenum(VI) to phosphorus(III).
|
| |
Chemistry,
11,
3255-3267.
|
 |
|
|
|
|
 |
A.Thapper,
A.Behrens,
J.Fryxelius,
M.H.Johansson,
F.Prestopino,
M.Czaun,
D.Rehder,
and
E.Nordlander
(2005).
Synthesis and characterization of molybdenum oxo complexes of two tripodal ligands: reactivity studies of a functional model for molybdenum oxotransferases.
|
| |
Dalton Trans,
(),
3566-3571.
|
 |
|
|
|
|
 |
E.Karakas,
and
C.Kisker
(2005).
Structural analysis of missense mutations causing isolated sulfite oxidase deficiency.
|
| |
Dalton Trans,
(),
3459-3463.
|
 |
|
|
|
|
 |
E.Karakas,
H.L.Wilson,
T.N.Graf,
S.Xiang,
S.Jaramillo-Busquets,
K.V.Rajagopalan,
and
C.Kisker
(2005).
Structural insights into sulfite oxidase deficiency.
|
| |
J Biol Chem,
280,
33506-33515.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
G.G.Barbier,
and
W.H.Campbell
(2005).
Viscosity effects on eukaryotic nitrate reductase activity.
|
| |
J Biol Chem,
280,
26049-26054.
|
 |
|
|
|
|
 |
M.Z.Seidahmed,
E.A.Alyamani,
M.S.Rashed,
A.A.Saadallah,
O.B.Abdelbasit,
M.M.Shaheed,
A.Rasheed,
F.A.Hamid,
and
M.A.Sabry
(2005).
Total truncation of the molybdopterin/dimerization domains of SUOX protein in an Arab family with isolated sulfite oxidase deficiency.
|
| |
Am J Med Genet A,
136,
205-209.
|
 |
|
|
|
|
 |
R.R.Mendel
(2005).
Molybdenum: biological activity and metabolism.
|
| |
Dalton Trans,
(),
3404-3409.
|
 |
|
|
|
|
 |
U.Kappler,
and
S.Bailey
(2005).
Molecular basis of intramolecular electron transfer in sulfite-oxidizing enzymes is revealed by high resolution structure of a heterodimeric complex of the catalytic molybdopterin subunit and a c-type cytochrome subunit.
|
| |
J Biol Chem,
280,
24999-25007.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
G.Bader,
M.Gomez-Ortiz,
C.Haussmann,
A.Bacher,
R.Huber,
and
M.Fischer
(2004).
Structure of the molybdenum-cofactor biosynthesis protein MoaB of Escherichia coli.
|
| |
Acta Crystallogr D Biol Crystallogr,
60,
1068-1075.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
H.L.Wilson,
and
K.V.Rajagopalan
(2004).
The role of tyrosine 343 in substrate binding and catalysis by human sulfite oxidase.
|
| |
J Biol Chem,
279,
15105-15113.
|
 |
|
|
|
|
 |
H.Mitsuhashi,
H.Ikeuchi,
S.Yamashita,
T.Kuroiwa,
Y.Kaneko,
K.Hiromura,
K.Ueki,
and
Y.Nojima
(2004).
Increased levels of serum sulfite in patients with acute pneumonia.
|
| |
Shock,
21,
99.
|
 |
|
|
|
|
 |
J.A.Hoy,
S.Kundu,
J.T.Trent,
S.Ramaswamy,
and
M.S.Hargrove
(2004).
The crystal structure of Synechocystis hemoglobin with a covalent heme linkage.
|
| |
J Biol Chem,
279,
16535-16542.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.A.Santamaria-Araujo,
B.Fischer,
T.Otte,
M.Nimtz,
R.R.Mendel,
V.Wray,
and
G.Schwarz
(2004).
The tetrahydropyranopterin structure of the sulfur-free and metal-free molybdenum cofactor precursor.
|
| |
J Biol Chem,
279,
15994-15999.
|
 |
|
|
|
|
 |
J.Kuper,
A.Llamas,
H.J.Hecht,
R.R.Mendel,
and
G.Schwarz
(2004).
Structure of the molybdopterin-bound Cnx1G domain links molybdenum and copper metabolism.
|
| |
Nature,
430,
803-806.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.Loschi,
S.J.Brokx,
T.L.Hills,
G.Zhang,
M.G.Bertero,
A.L.Lovering,
J.H.Weiner,
and
N.C.Strynadka
(2004).
Structural and biochemical identification of a novel bacterial oxidoreductase.
|
| |
J Biol Chem,
279,
50391-50400.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.H.Stipanuk
(2004).
Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine.
|
| |
Annu Rev Nutr,
24,
539-577.
|
 |
|
|
|
|
 |
U.Kappler,
and
S.Bailey
(2004).
Crystallization and preliminary X-ray analysis of sulfite dehydrogenase from Starkeya novella.
|
| |
Acta Crystallogr D Biol Crystallogr,
60,
2070-2072.
|
 |
|
|
|
|
 |
X.Zhang,
A.S.Vincent,
B.Halliwell,
and
K.P.Wong
(2004).
A mechanism of sulfite neurotoxicity: direct inhibition of glutamate dehydrogenase.
|
| |
J Biol Chem,
279,
43035-43045.
|
 |
|
|
|
|
 |
C.Feng,
H.L.Wilson,
J.K.Hurley,
J.T.Hazzard,
G.Tollin,
K.V.Rajagopalan,
and
J.H.Enemark
(2003).
Role of conserved tyrosine 343 in intramolecular electron transfer in human sulfite oxidase.
|
| |
J Biol Chem,
278,
2913-2920.
|
 |
|
|
|
|
 |
C.Feng,
H.L.Wilson,
J.K.Hurley,
J.T.Hazzard,
G.Tollin,
K.V.Rajagopalan,
and
J.H.Enemark
(2003).
Essential role of conserved arginine 160 in intramolecular electron transfer in human sulfite oxidase.
|
| |
Biochemistry,
42,
12235-12242.
|
 |
|
|
|
|
 |
H.K.Joshi,
J.J.Cooney,
F.E.Inscore,
N.E.Gruhn,
D.L.Lichtenberger,
and
J.H.Enemark
(2003).
Investigation of metal-dithiolate fold angle effects: implications for molybdenum and tungsten enzymes.
|
| |
Proc Natl Acad Sci U S A,
100,
3719-3724.
|
 |
|
|
|
|
 |
M.J.Rudolph,
J.L.Johnson,
K.V.Rajagopalan,
and
C.Kisker
(2003).
The 1.2 A structure of the human sulfite oxidase cytochrome b(5) domain.
|
| |
Acta Crystallogr D Biol Crystallogr,
59,
1183-1191.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.L.Johnson,
K.V.Rajagopalan,
W.O.Renier,
I.Van der Burgt,
and
W.Ruitenbeek
(2002).
Isolated sulfite oxidase deficiency: mutation analysis and DNA-based prenatal diagnosis.
|
| |
Prenat Diagn,
22,
433-436.
|
 |
|
|
|
|
 |
R.Hille
(2002).
Molybdenum and tungsten in biology.
|
| |
Trends Biochem Sci,
27,
360-367.
|
 |
|
|
|
|
 |
W.Mifsud,
and
A.Bateman
(2002).
Membrane-bound progesterone receptors contain a cytochrome b5-like ligand-binding domain.
|
| |
Genome Biol,
3,
RESEARCH0068.
|
 |
|
|
|
|
 |
T.Eilers,
G.Schwarz,
H.Brinkmann,
C.Witt,
T.Richter,
J.Nieder,
B.Koch,
R.Hille,
R.Hänsch,
and
R.R.Mendel
(2001).
Identification and biochemical characterization of Arabidopsis thaliana sulfite oxidase. A new player in plant sulfur metabolism.
|
| |
J Biol Chem,
276,
46989-46994.
|
 |
|
|
|
|
 |
C.E.Stevenson,
F.Sargent,
G.Buchanan,
T.Palmer,
and
D.M.Lawson
(2000).
Crystal structure of the molybdenum cofactor biosynthesis protein MobA from Escherichia coli at near-atomic resolution.
|
| |
Structure,
8,
1115-1125.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.Kuper,
T.Palmer,
R.R.Mendel,
and
G.Schwarz
(2000).
Mutations in the molybdenum cofactor biosynthetic protein Cnx1G from Arabidopsis thaliana define functions for molybdopterin binding, molybdenum insertion, and molybdenum cofactor stabilization.
|
| |
Proc Natl Acad Sci U S A,
97,
6475-6480.
|
 |
|
|
|
|
 |
M.M.Wuebbens,
M.T.Liu,
K.Rajagopalan,
and
H.Schindelin
(2000).
Insights into molybdenum cofactor deficiency provided by the crystal structure of the molybdenum cofactor biosynthesis protein MoaC.
|
| |
Structure,
8,
709-718.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.T.Liu,
M.M.Wuebbens,
K.V.Rajagopalan,
and
H.Schindelin
(2000).
Crystal structure of the gephyrin-related molybdenum cofactor biosynthesis protein MogA from Escherichia coli.
|
| |
J Biol Chem,
275,
1814-1822.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.C.Bray,
B.Adams,
A.T.Smith,
B.Bennett,
and
S.Bailey
(2000).
Reversible dissociation of thiolate ligands from molybdenum in an enzyme of the dimethyl sulfoxide reductase family.
|
| |
Biochemistry,
39,
11258-11269.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.D.Garton,
C.A.Temple,
I.K.Dhawan,
M.J.Barber,
K.V.Rajagopalan,
and
M.K.Johnson
(2000).
Resonance Raman characterization of biotin sulfoxide reductase. Comparing oxomolybdenum enzymes in the ME(2)SO reductase family.
|
| |
J Biol Chem,
275,
6798-6805.
|
 |
|
|
|
|
 |
U.Kappler,
B.Bennett,
J.Rethmeier,
G.Schwarz,
R.Deutzmann,
A.G.McEwan,
and
C.Dahl
(2000).
Sulfite:Cytochrome c oxidoreductase from Thiobacillus novellus. Purification, characterization, and molecular biology of a heterodimeric member of the sulfite oxidase family.
|
| |
J Biol Chem,
275,
13202-13212.
|
 |
|
|
|
|
 |
B.Adams,
A.T.Smith,
S.Bailey,
A.G.McEwan,
and
R.C.Bray
(1999).
Reactions of dimethylsulfoxide reductase from Rhodobacter capsulatus with dimethyl sulfide and with dimethyl sulfoxide: complexities revealed by conventional and stopped-flow spectrophotometry.
|
| |
Biochemistry,
38,
8501-8511.
|
 |
|
|
|
|
 |
D.J.Richardson,
and
N.J.Watmough
(1999).
Inorganic nitrogen metabolism in bacteria.
|
| |
Curr Opin Chem Biol,
3,
207-219.
|
 |
|
|
|
|
 |
J.C.Hilton,
C.A.Temple,
and
K.V.Rajagopalan
(1999).
Re-design of Rhodobacter sphaeroides dimethyl sulfoxide reductase. Enhancement of adenosine N1-oxide reductase activity.
|
| |
J Biol Chem,
274,
8428-8436.
|
 |
|
|
|
|
 |
J.M.Dias,
M.E.Than,
A.Humm,
R.Huber,
G.P.Bourenkov,
H.D.Bartunik,
S.Bursakov,
J.Calvete,
J.Caldeira,
C.Carneiro,
J.J.Moura,
I.Moura,
and
M.J.Romão
(1999).
Crystal structure of the first dissimilatory nitrate reductase at 1.9 A solved by MAD methods.
|
| |
Structure,
7,
65-79.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.C.Edwards,
J.L.Johnson,
B.Marriage,
T.N.Graf,
K.E.Coyne,
K.V.Rajagopalan,
and
I.M.MacDonald
(1999).
Isolated sulfite oxidase deficiency: review of two cases in one family.
|
| |
Ophthalmology,
106,
1957-1961.
|
 |
|
|
|
|
 |
M.S.Brody,
and
R.Hille
(1999).
The kinetic behavior of chicken liver sulfite oxidase.
|
| |
Biochemistry,
38,
6668-6677.
|
 |
|
|
|
|
 |
W.H.Campbell
(1999).
NITRATE REDUCTASE STRUCTURE, FUNCTION AND REGULATION: Bridging the Gap between Biochemistry and Physiology.
|
| |
Annu Rev Plant Physiol Plant Mol Biol,
50,
277-303.
|
 |
|
|
|
|
 |
C.Kisker,
H.Schindelin,
D.Baas,
J.Rétey,
R.U.Meckenstock,
and
P.M.Kroneck
(1998).
A structural comparison of molybdenum cofactor-containing enzymes.
|
| |
FEMS Microbiol Rev,
22,
503-521.
|
 |
|
|
|
|
 |
J.McMaster,
and
J.H.Enemark
(1998).
The active sites of molybdenum- and tungsten-containing enzymes.
|
| |
Curr Opin Chem Biol,
2,
201-207.
|
 |
|
|
|
|
 |
K.Klarskov,
F.Verté,
G.Van Driessche,
T.E.Meyer,
M.A.Cusanovich,
and
J.Van Beeumen
(1998).
The primary structure of soluble cytochrome c-551 from the phototrophic green sulfur bacterium Chlorobium limicola, strain Tassajara, reveals a novel c-type cytochrome.
|
| |
Biochemistry,
37,
10555-10562.
|
 |
|
|
|
|
 |
R.Hille,
J.Rétey,
U.Bartlewski-Hof,
and
W.Reichenbecher
(1998).
Mechanistic aspects of molybdenum-containing enzymes.
|
| |
FEMS Microbiol Rev,
22,
489-501.
|
 |
|
|
|
|
 |
R.M.Garrett,
J.L.Johnson,
T.N.Graf,
A.Feigenbaum,
and
K.V.Rajagopalan
(1998).
Human sulfite oxidase R160Q: identification of the mutation in a sulfite oxidase-deficient patient and expression and characterization of the mutant enzyme.
|
| |
Proc Natl Acad Sci U S A,
95,
6394-6398.
|
 |
|
|
|
|
 |
U.Ermler,
W.Grabarse,
S.Shima,
M.Goubeaud,
and
R.K.Thauer
(1998).
Active sites of transition-metal enzymes with a focus on nickel.
|
| |
Curr Opin Struct Biol,
8,
749-758.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

