 |
CoFactor: Nicotinamide-adenine dinucleotideGeneral information2D representation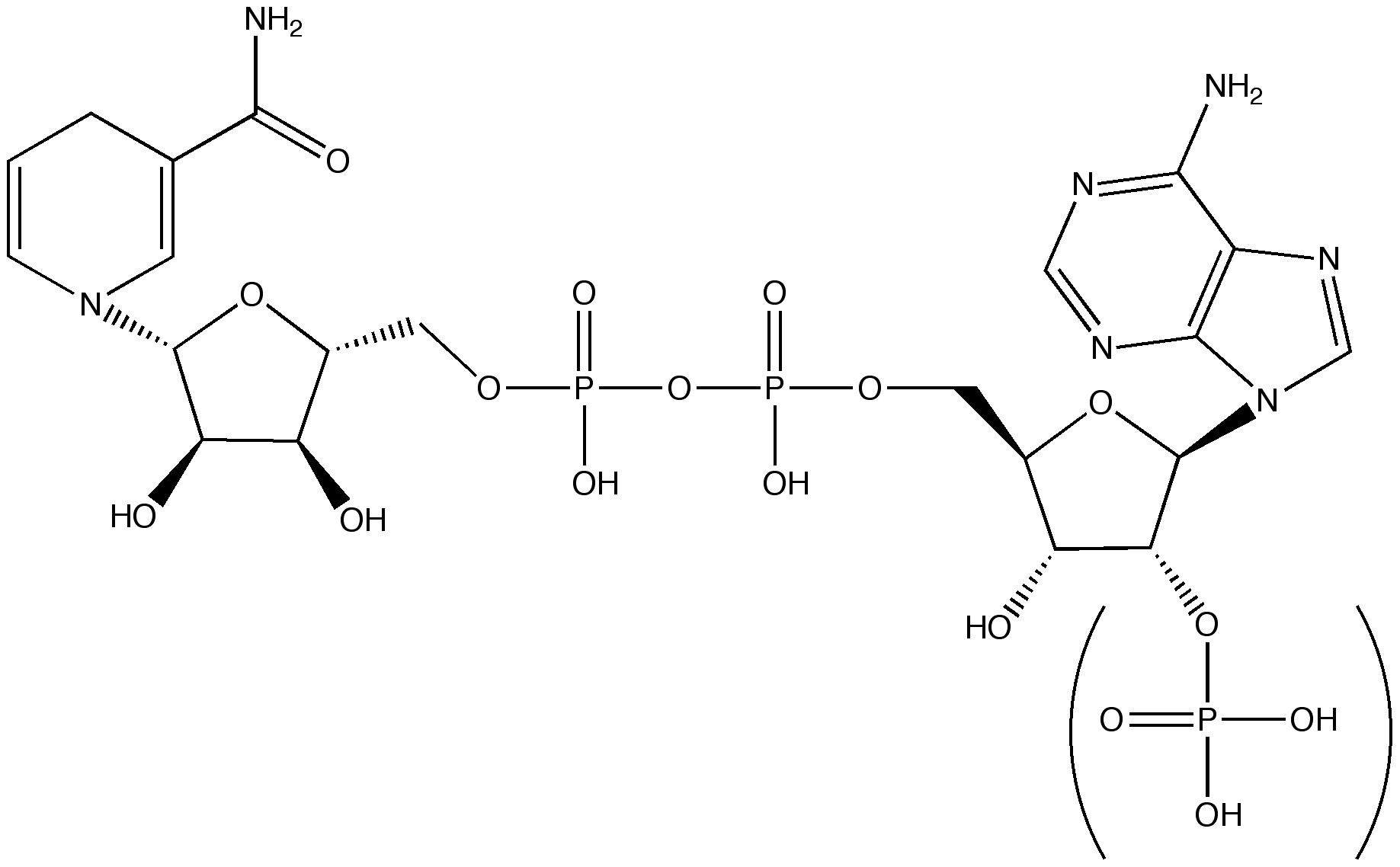
Key facts
TagsMolecular functionNAD(P) assists in hydride transfers [2]. Therefore the cofactor exists in two states: NAD(P)+ and NAD(P)H/H+. In addition to its catalytic function, NAD(P) is also involved in regulation. NAD levels in the cell influence transcriptional reprogramming and regulate physiological functions of a cell in response to perturbations in NAD(H) levels to maintain homeostatic conditions [3]. Chemical propertiesThe nicotinamide ring (pyridine ring) is planar in the oxidised form but distorted (boat conformation) in the reduced form [1]. This is not always visible in the PDB structures because some of the reduced NADs have been subjected to a refinement algorithm that uses standard planar restraints on the cofactor [1]. The double bond between C5 and C6 is weakened upon adduct formation.[2] The hydride transfer mechanism involving NAD(P) cofactors is accompanied by the transfer of a proton. The coupling between hydride and proton transfer has to be well orchestrated to prevent the hydride ion and proton to form a hydrogen molecule [1]. PathwaysNAD is involved in DNA repair, calcium-dependent signalling pathways and lifespan extension in yeast [4]. CommentNAD is the most abundant electron carrier in cell metabolism [2]. NAD is an essential cofactor for both energy metabolism and signal transduction [4]. For some enzymes, NAD is not a coenzyme (dissociating from the protein after each catalytic cycle) but a prosthetic group (that remains bound to the enzyme and does not exchange with external NAD)[6]. Due to the dependency of human cells on the nutritional intake of vitamin B3, the biosynthetic enzymes are antibiotic drug targets [5],[4]. References
 |

