Glutathione S-transferase A
Glutathione transferase (GST) is a detoxification enzyme found in the liver. GSTs are the most important enzymes involved in the metabolism of electrophilic xenobiotic/endobiotic compounds. These enzymes are able to catalyze the nucleophilic addition of glutathione (GSH) sulfur thiolate to a wide range of electrophilic substrates, building up a less toxic and more soluble compound. Classes alpha, pi, and mu are the most extensively studied GSTs. GSTA and GSTP are similar in active site residues and have the same mechanism for glutathione transfer.
Reference Protein and Structure
- Sequence
-
P09211
 (2.5.1.18)
(2.5.1.18)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Homo sapiens (Human)

- PDB
-
1pgt
- CRYSTAL STRUCTURE OF HUMAN GLUTATHIONE S-TRANSFERASE P1-1[V104] COMPLEXED WITH S-HEXYLGLUTATHIONE
(1.8 Å)



- Catalytic CATH Domains
-
3.40.30.10
 (see all for 1pgt)
(see all for 1pgt)
Enzyme Reaction (EC:2.5.1.18)
Enzyme Mechanism
Introduction
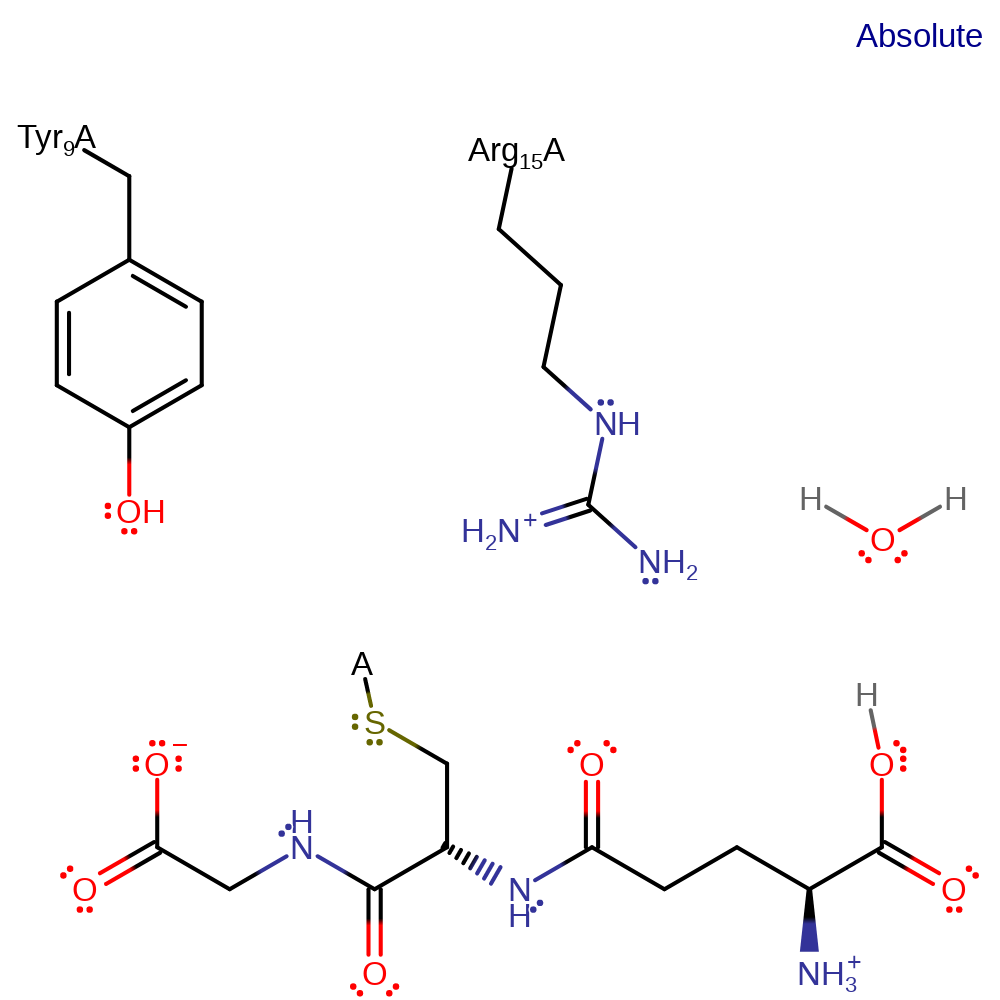
GSH transfers its GSH-SH proton onto its GSH-COO- through a proton transfer mediated by a water molecule. A water molecule deprotonates the thiolate and brigdes the proton to the carboxylate part of the GSH molecule. This reaction activates GSH to attack the electrophilic substrate being metabolised. The Tyr9 and Arg15 stabilize the GSH and the transition state.
Catalytic Residues Roles
| UniProt | PDB* (1pgt) | ||
| Arg15 | Arg15A | Arg15 is crucial for stabilising the GSH thiolate when it turns into a nucleophile. Without this arginine the activity drops 200 to 400 times. | electrostatic stabiliser |
| Tyr9 | Tyr9A | Tyr9 modifies the pKa of the thiolate of the GSH. This helps deprotonate the GSH thiolate with a water molecule, activating GSH. | modifies pKa, hydrogen bond donor |
Chemical Components
intermediate formation, proton transfer, bimolecular nucleophilic substitution, intermediate collapse, overall product formed, overall reactant usedReferences
- Dourado DF et al. (2010), J Phys Chem B, 114, 12972-12980. Glutathione transferase classes alpha, pi, and mu: GSH activation mechanism. DOI:10.1021/jp1053875. PMID:20853826.
- Dourado DF et al. (2013), Biochemistry, 52, 8069-8078. Mechanism of glutathione transferase P1-1-catalyzed activation of the prodrug canfosfamide (TLK286, TELCYTA). DOI:10.1021/bi4005705. PMID:24066958.
- Dourado DFAR et al. (2010), J Phys Chem B, 114, 1690-1697. Glutathione Transferase A1-1: Catalytic Importance of Arginine 15. DOI:https://doi.org/10.1021/jp908251z.
- Grahn E et al. (2006), Acta Crystallogr D Biol Crystallogr, 62, 197-207. New crystal structures of human glutathione transferase A1-1 shed light on glutathione binding and the conformation of the C-terminal helix. DOI:10.1107/S0907444905039296. PMID:16421451.

Step 1. Tyr9 lowers the pKa of the thiolate and a water molecule deprotonates the thiolate bridging the proton to the GSH-COO- part of the molecule.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg15A | electrostatic stabiliser |
| Tyr9A | modifies pKa, hydrogen bond donor |
Chemical Components
intermediate formation, proton transfer
Step 2. The hydronium intermediate collapses and the GSH sulphur atom nucleophilically attacks the electrophile which is being metabolised.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg15A | electrostatic stabiliser |
| Tyr9A | modifies pKa, hydrogen bond donor |
Chemical Components
proton transfer, ingold: bimolecular nucleophilic substitution, intermediate collapse, overall product formed, overall reactant usedIntroduction
This is the mechanism of the activation of the prodrug canfosfamide. A proton is transferred from the Tyr7 hydroxyl group to the canfosfamide COO- oxygen atom via a water molecule bridge. Then the negatively charged Tyr7 works as a base and receives a proton from the substrates α-C atom. Lastly, we observe the breaking of the bond between canfosfamides β-C and the adjacent oxygen and the consequent release of the phosphorodiamidate.
Catalytic Residues Roles
| UniProt | PDB* (1pgt) | ||
| Tyr8 | Tyr7(8)A | Tyr7 is deprotonated by the COO- of canfosfamide via a water molecule bridge. The deprotonated Tyr7 then deprotonates the substrates α-C atom causing it to break apart into two products. | proton acceptor, proton donor |
Chemical Components
proton transfer, native state of enzyme regenerated, unimolecular elimination by the conjugate base, overall product formed, intramolecular rearrangementReferences
- Dourado DF et al. (2013), Biochemistry, 52, 8069-8078. Mechanism of glutathione transferase P1-1-catalyzed activation of the prodrug canfosfamide (TLK286, TELCYTA). DOI:10.1021/bi4005705. PMID:24066958.
- Dourado DF et al. (2010), J Phys Chem B, 114, 12972-12980. Glutathione transferase classes alpha, pi, and mu: GSH activation mechanism. DOI:10.1021/jp1053875. PMID:20853826.
- Ji X (1997),CRYSTAL STRUCTURE OF HUMAN GLUTATHIONE S-TRANSFERASE P1-1[V104] COMPLEXED WITH S-HEXYLGLUTATHIONE. DOI:10.2210/pdb1PGT/pdb.

Step 1. Tyr7 gets deprotonated by the canfosfamide COO- end through a water bridge molecule.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Tyr7(8)A | proton donor |
Chemical Components
proton transfer
Step 2. Tyr7 works as a base and receives a proton from canfosfamides α-C atom.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Tyr7(8)A | proton acceptor |
Chemical Components
proton transfer, native state of enzyme regenerated
Step 3. The bond between canfosfamide β-C and the adjacent oxygen breaks and consequentially releases the phosphorodiamidate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|





 Download:
Download:  Download:
Download: