Polyamine oxidase (propane-1,3-diamine-forming)
Polyamine oxidase is able to catalyse the oxidation of the secondary amino groups of polyamines to their corresponding imino forms, using FAD as a cofactor coupled to eventual reduction of H2O2 to form water. The product of the reaction depends on the initial starting material; for example mammalian polyamine oxidase can convert spermidine to putrescine. Polyamines bind DNA and regulate transcription and translation, thus play roles in cell differentiation and multiplication, causing them to be implicated in the development of certain forms of cancer. As a result the enzyme is of interest as a drug target. Despite different physiological roles, mammalian, plant and bacterial forms of the enzyme show significant sequence and structural homology, and polyamine oxidases also show homology to monoamine oxidases, suggesting a common catalytic mechanism.
Reference Protein and Structure
- Sequence
-
O64411
 (1.5.3.14, 1.5.3.15)
(1.5.3.14, 1.5.3.15)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Zea mays (Maize)

- PDB
-
1b5q
- A 30 ANGSTROM U-SHAPED CATALYTIC TUNNEL IN THE CRYSTAL STRUCTURE OF POLYAMINE OXIDASE
(1.9 Å)



- Catalytic CATH Domains
-
3.50.50.60
 (see all for 1b5q)
(see all for 1b5q)
- Cofactors
- Fadh2(2-) (1)
Enzyme Mechanism
Introduction
The reaction is believed to proceed through nucleophilic attack from the amino group, deprotonated by Glu 62, on the C4a of FAD. This leads to a covalent adduct which collapses, assisted by proton transfer from the alpha carbon to the N5 of FAD via a water molecule, to give the product imine.
Catalytic Residues Roles
| UniProt | PDB* (1b5q) | ||
| Glu90 | Glu62C | Acts as general base to remove proton from the nucleophilic amino group of the substrate. | proton acceptor, proton donor |
Chemical Components
proton transfer, bimolecular nucleophilic addition, intermediate formation, elimination (not covered by the Ingold mechanisms), intermediate collapse, inferred reaction step, overall product formed, native state of enzyme regeneratedReferences
- Royo M et al. (2005), Biochemistry, 44, 7079-7084. Mechanistic Studies of Mouse Polyamine Oxidase with N1,N12-Bisethylspermine as a Substrate†. DOI:10.1021/bi050347k. PMID:15865452.
- Binda C et al. (2001), Biochemistry, 40, 2766-2776. Structural Bases for Inhibitor Binding and Catalysis in Polyamine Oxidase†,‡. DOI:10.1021/bi002751j. PMID:11258887.
- Binda C et al. (1999), Structure, 7, 265-276. A 30 Å long U-shaped catalytic tunnel in the crystal structure of polyamine oxidase. DOI:10.1016/S0969-2126(99)80037-9.

Step 1. Glu62 deprotonates the secondary amine nitrogen of the reactant.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu62C | proton acceptor |
Chemical Components
proton transfer
Step 2. Nucleophilic attack of the secondary amine on C4 of FAD is accompanied by proton transfer to N5 of FAD, forming a covalent intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|
Chemical Components
ingold: bimolecular nucleophilic addition, proton transfer, intermediate formation
Step 3. The reactant-FAD bond is cleaved, forming FADH- and an imine intermediate. FADH- is reoxidised by molecular oxygen.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|
Chemical Components
elimination (not covered by the Ingold mechanisms), intermediate formation
Step 4. The water molecule that is H bonded to the flavin N5 atom is well positioned to perform the hydrolytic attack on the imino compound.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|
Chemical Components
ingold: bimolecular nucleophilic addition, intermediate formation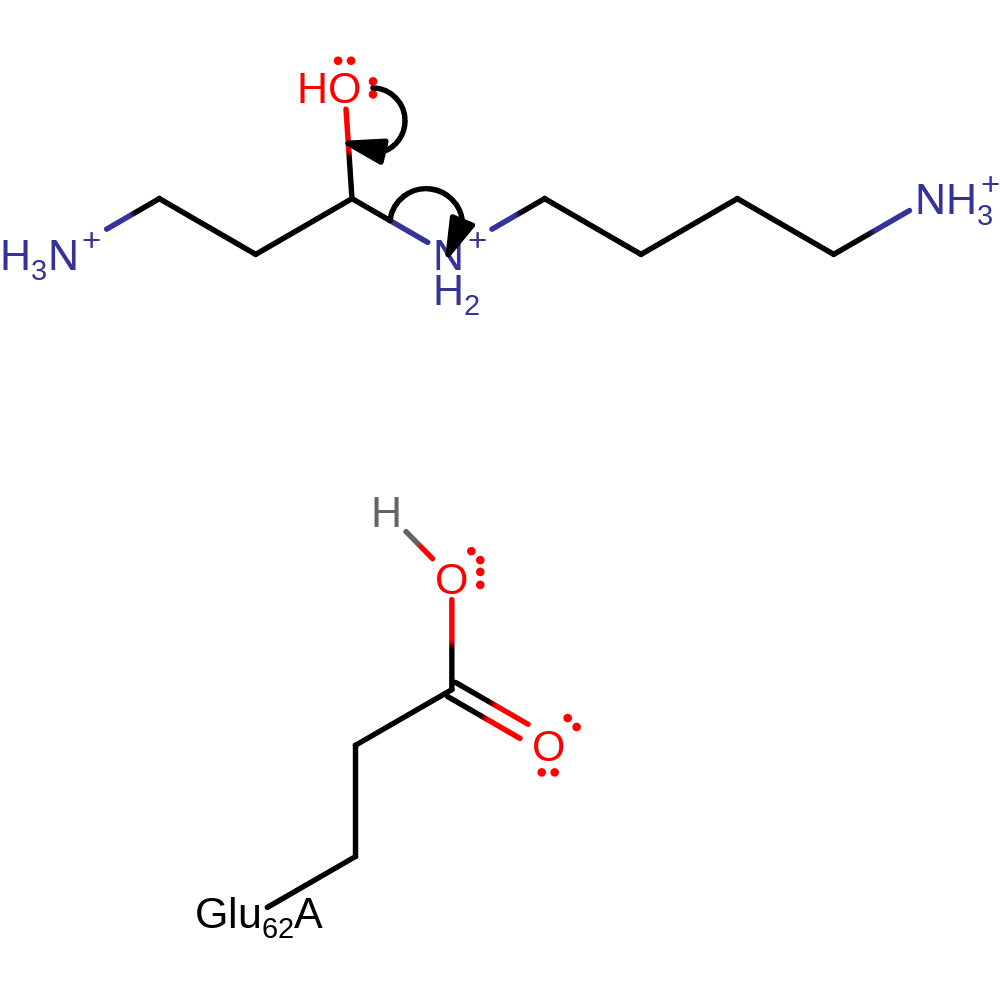
Step 5. Proton transfer between the hydroxyl group and secondary amine occurs prior to this step. The amine is then eliminated, forming the aldehyde product.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|
Chemical Components
elimination (not covered by the Ingold mechanisms), intermediate collapse, inferred reaction step, overall product formedCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu62C | proton donor |







 Download:
Download: