Streptopain
Cysteine protease which is a virulance factor for many infectious diseases. It displays structural homology with papains despite having such low sequence identity with the superfamily that it merits being placed in a completely separate class.
Reference Protein and Structure
- Sequence
-
P0C0J0
 (3.4.22.10)
(3.4.22.10)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Streptococcus pyogenes (Bacteria)

- PDB
-
1dki
- CRYSTAL STRUCTURE OF THE ZYMOGEN FORM OF STREPTOCOCCAL PYROGENIC EXOTOXIN B ACTIVE SITE (C47S) MUTANT
(1.6 Å)



- Catalytic CATH Domains
-
3.30.910.30
 3.90.70.50
3.90.70.50  (see all for 1dki)
(see all for 1dki)
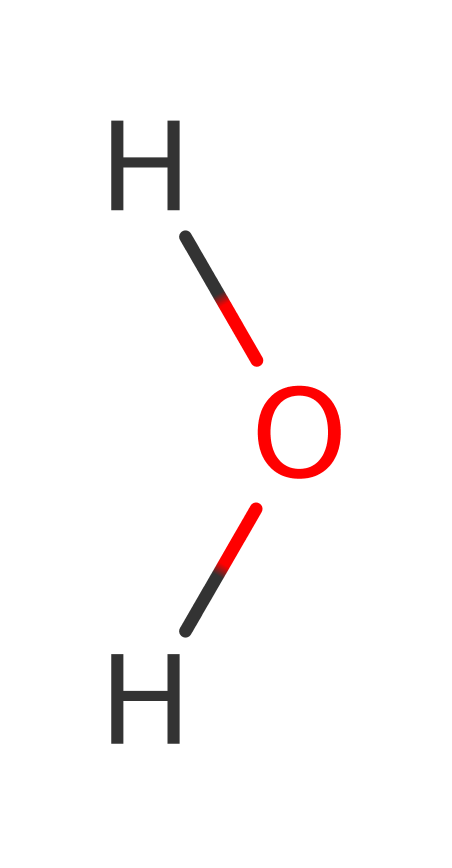
Enzyme Reaction (EC:3.4.22.10)
+
→
+
Alternative enzyme names: Streptococcus peptidase A, Streptococcus protease, Streptococcal cysteine proteinase, Streptococcal proteinase,
Enzyme Mechanism
Introduction
Cys 47 acts as the nucleophile for initial attack on the peptide substrate. The tetrahedral intermediate then collapses on protonation of the leaving group by His 195. This leaves an acyl-enzyme intermediate which is hydrolysed by an activated water molecule to complete the reaction.
Catalytic Residues Roles
| UniProt | PDB* (1dki) | ||
| Trp357 | Trp212(330)A | Stabilises the thiolate-imidazolium pair by interacting with His 340. | |
| Cys192 (main-N) | Ser47(165)A (main-N) | Amide backbone forms part of the oxyanion hole which functions to stabilise the negative charge that accumulates on the carbonyl oxygen of the substrate during the reaction. | |
| His340 | His195(313)A | Protonates leaving group to facilitate collapse of tetrahedral intermediate, then activates water to allow hydrolysis of acyl enzyme intermediate. | proton shuttle (general acid/base) |
| Cys192 | Ser47(165)A | Cys 47 (appears as Ser 47 in pdb file) acts as nucleophile for initial attack on the peptide substrate to form an acyl enzyme intermediate. Also stabilises the tetrahedral intermediate via oxyanion hole. | covalently attached, electrostatic stabiliser |
*PDB label guide - RESx(y)B(C) - RES: Residue Name; x: Residue ID in PDB file;
y: Residue ID in PDB sequence if different from PDB file; B: PDB Chain;
C: Biological Assembly Chain if different from PDB. If label is "Not Found" it means this residue is not found in the reference PDB.
Chemical Components
References
- Kagawa TF et al. (2000), Proc Natl Acad Sci U S A, 97, 2235-2240. Crystal structure of the zymogen form of the group A Streptococcus virulence factor SpeB: An integrin-binding cysteine protease. DOI:10.1073/pnas.040549997. PMID:10681429.
- Olsen JG et al. (2009), J Mol Biol, 393, 693-703. Structure of the mature Streptococcal cysteine protease exotoxin mSpeB in its active dimeric form. DOI:10.1016/j.jmb.2009.08.046. PMID:19712682.
- Gubba S et al. (2000), Infect Immun, 68, 3716-3719. Replacement of Histidine 340 with Alanine Inactivates the Group A Streptococcus Extracellular Cysteine Protease Virulence Factor. DOI:10.1128/iai.68.6.3716-3719.2000. PMID:10816533.
Catalytic Residues Roles
| Residue | Roles |
|---|---|
| Ser47(165)A | electrostatic stabiliser, covalently attached |
| His195(313)A | proton shuttle (general acid/base) |