Ribonuclease V
Angiogenin is a ribonuclease which induces neovascularisation. It binds specifically to endothelial cells in culture and elicits second-messenger responses. It also binds heparin and can serve as a substratum for endothelial cell adhesion. Its amino acid sequence is 33% identical to that of bovine pancreatic ribonuclease RNase A and it has the same general catalytic properties as RNase A, however, it differs markedly both in magnitude and in specificity with RNase A.
Angiogenin was first isolated from culture medium conditioned by adenocarcinoma cells, and has since been shown to be critical for the establishment and/or metastatic spread of a wide variety of human tumours in athymic mice, most likely by supporting the growth of tumour vasculature. Moreover, clinical studies have revealed increased Angiogenin expression to be associated with progression of several human cancers. These findings identify Angiogenin as a promising target for new anticancer drugs.
Reference Protein and Structure
- Sequences
-
P13489

P03950 (3.1.27.-)
(3.1.27.-)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Homo sapiens (Human)

- PDB
-
1a4y
- RIBONUCLEASE INHIBITOR-ANGIOGENIN COMPLEX
(2.0 Å)



- Catalytic CATH Domains
-
3.10.130.10
 (see all for 1a4y)
(see all for 1a4y)
Enzyme Mechanism
Introduction
The cleavage of substrate by ribonucleases is a two-step reaction: the first step is a transesterification of which a nucleophilic displacement at the phosphorus atom of the 5' leaving group by the 2' entering oxygen atom takes place, forming a nucleoside 2',3'-cyclophosphate intermediate, which is hydrolysed in the second step to yield the product. His13 acts as a base to abstract a proton from 2'-oxygen of a substrate molecule, and thereby facilitates its attack on the phophorus atom. This attack proceeds inline to displace a nucleoside. His114 acts as an acid to protonate the 5''-oxygen to facilitate its displacement. The subsequent hydrolysis resembles the reverse of transphosphorylation where His114 activates a water molecule by deprotonation to facilitate its nucleophilic attack. Lys40 stabilises the transition state.
Catalytic Residues Roles
| UniProt | PDB* (1a4y) | ||
| His37 | His13B | It abstracts a proton from 2'-oxygen of a substrate molecule, and thereby facilitates its attack on the phosphorous atom and acts as a base in the subsequent hydrolysis. | increase nucleophilicity, promote heterolysis, proton acceptor, proton donor |
| Lys64 | Lys40B | It stabilises the transition state. | electrostatic stabiliser |
| His138 | His114B | It acts as an acid to protonate the 5''-oxygen to facilitate its displacement of the leaving group. It activates a water nucleophile by deprotonation. | increase nucleophilicity, promote heterolysis, proton acceptor, proton donor |
Chemical Components
cyclisation, intramolecular nucleophilic addition, proton transfer, overall reactant used, overall product formed, native state of enzyme regenerated, bimolecular nucleophilic substitution, decyclisationReferences
- Shapiro R et al. (1989), Biochemistry, 28, 1726-1732. Role of lysines in human angiogenin: chemical modification and site-directed mutagenesis. DOI:10.1021/bi00430a045. PMID:2497770.
- Chatzileontiadou DS et al. (2016), FEBS Lett, 590, 3005-3018. The ammonium sulfate inhibition of human angiogenin. DOI:10.1002/1873-3468.12335. PMID:27483019.
- Leonidas DD et al. (2001), Protein Sci, 10, 1669-1676. Binding of phosphate and pyrophosphate ions at the active site of human angiogenin as revealed by X-ray crystallography. DOI:10.1110/ps.13601. PMID:11468363.
- Shapiro R et al. (1989), Biochemistry, 28, 7401-7408. Site-directed mutagenesis of histidine-13 and histidine-114 of human angiogenin. Alanine derivatives inhibit angiogenin-induced angiogenesis. DOI:10.1021/bi00444a038. PMID:2479414.
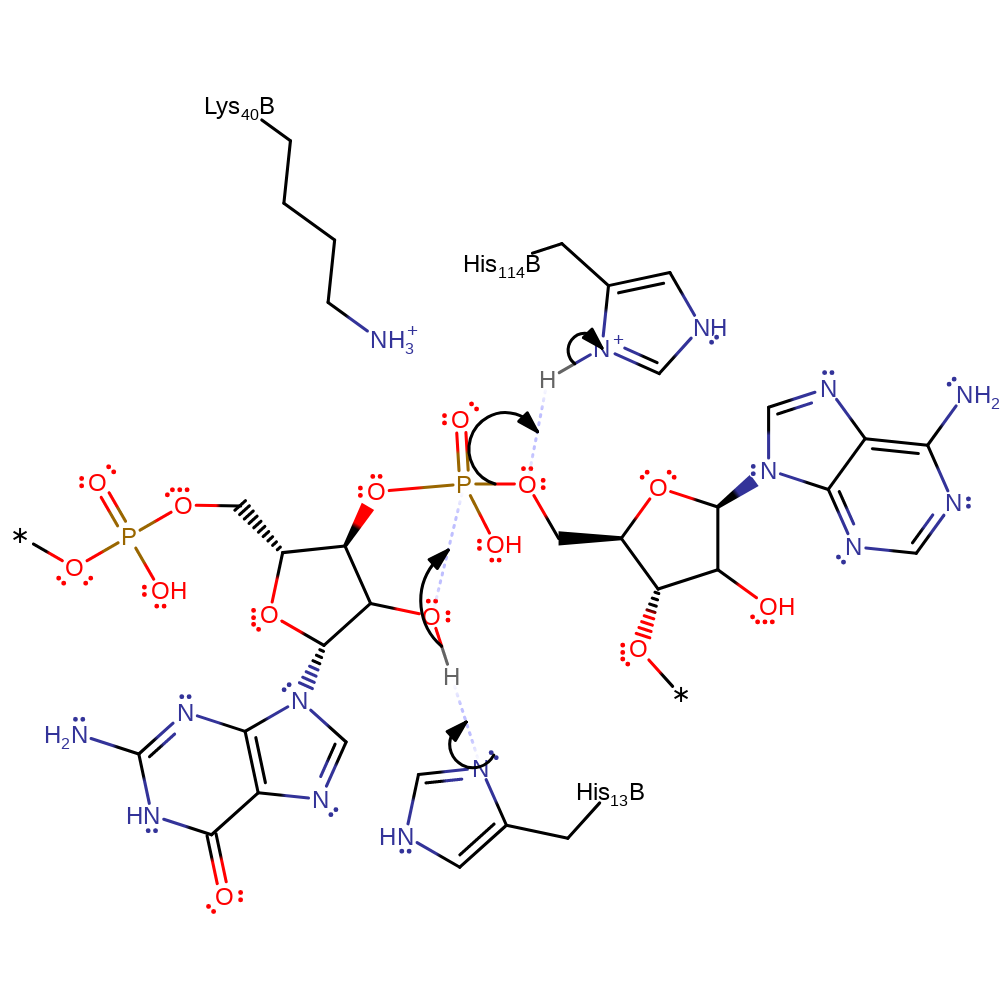
Step 1. His13 deprotonates the 2' hydroxyl group this promotes its nucleophilic attack on the phosphate group forming a cyclic intermediate. His114 protonates the the 5' hydroxyl leaving group promoting the cleavage.This SN2 reaction proceeds via a penta-coordinate transition state stabilized by Lys40.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Lys40B | electrostatic stabiliser |
| His13B | increase nucleophilicity |
| His114B | promote heterolysis |
| His13B | proton acceptor |
| His114B | proton donor |
Chemical Components
cyclisation, ingold: intramolecular nucleophilic addition, proton transfer, overall reactant used, overall product formed
Step 2. His114 now acting as a base deprotonates a water molecule allowing it to perform a nucleophillic attack on the phosphate group. This leads to the cleavage of the 2' hydroxyl- phosphate bond upon protonation by His13.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His13B | promote heterolysis |
| His114B | increase nucleophilicity |
| Lys40B | electrostatic stabiliser |
| His13B | proton donor |
| His114B | proton acceptor |



 Download:
Download: