Carbonate dehydratase (beta class)
Beta-carbonic anhydrases are found in a variety of higher plants, simple eukaryotes, eubacteria and archaea. they catalyse the interconversion of carbon dioxide and bicarbonate. These are zinc-containing metalloenzymes.
Reference Protein and Structure
- Sequence
-
P61517
 (4.2.1.1)
(4.2.1.1)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Escherichia coli K-12 (Bacteria)

- PDB
-
1i6p
- CRYSTAL STRUCTURE OF E. COLI BETA CARBONIC ANHYDRASE (ECCA)
(2.0 Å)



- Catalytic CATH Domains
-
3.40.1050.10
 (see all for 1i6p)
(see all for 1i6p)
- Cofactors
- Zinc(2+) (1)
Enzyme Reaction (EC:4.2.1.1)
Enzyme Mechanism
Introduction
After association of carbon dioxide with the binding site, Asp 44 (activated by binding with zinc and electrostatic interaction with Arg 46) acts as a base to abstract a proton from the water molecule. This yields a nucleophilic hydroxide that binds the zinc which further activates it for nucleophilic attack of the carbon dioxide to generate zinc-bound bicarbonate. Loss of the proton is catalysed by Asp 44 again acting as a base to transfer the proton to the solvent, and the product leaves the active site.
Catalytic Residues Roles
| UniProt | PDB* (1i6p) | ||
| Cys42, Cys101, His98 | Cys42A, Cys101A, His98A | Coordinate the zinc ion | metal ligand |
| Asp44 | Asp44A | Acts as a base to deprotonate the attacking water nucleophile and the substrate. | metal ligand, proton acceptor |
| Arg46 | Arg46A | Activates Asp 44. | increase basicity, electrostatic stabiliser |
Chemical Components
proton transfer, coordination, bimolecular nucleophilic addition, overall reactant used, overall product formed, decoordination from a metal ionReferences
- Smith KS et al. (2002), J Bacteriol, 184, 4240-4245. Roles of the Conserved Aspartate and Arginine in the Catalytic Mechanism of an Archaeal -Class Carbonic Anhydrase. DOI:10.1128/jb.184.15.4240-4245.2002. PMID:12107142.
- Rowlett RS (2014), Subcell Biochem, 75, 53-76. Structure and catalytic mechanism of β-carbonic anhydrases. DOI:10.1007/978-94-007-7359-2_4. PMID:24146374.
- Cronk JD et al. (2001), Protein Sci, 10, 911-922. Crystal structure of E. coli β-carbonic anhydrase, an enzyme with an unusual pH-dependent activity. DOI:10.1110/ps.46301. PMID:11316870.
- Mitsuhashi S et al. (2000), J Biol Chem, 275, 5521-5526. X-ray Structure of β-Carbonic Anhydrase from the Red Alga, Porphyridium purpureum , Reveals a Novel Catalytic Site for CO2 Hydration . DOI:10.1074/jbc.275.8.5521. PMID:10681531.

Step 1. Asp44, activated by electrostatic interactions with zinc and Arg46, acts as a base to abstract a proton from the water. This generates a hydroxide ion which can bind to the zinc.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg46A | electrostatic stabiliser |
| Cys42A | metal ligand |
| Asp44A | metal ligand |
| His98A | metal ligand |
| Cys101A | metal ligand |
| Arg46A | increase basicity |
| Asp44A | proton acceptor |
Chemical Components
proton transfer, coordination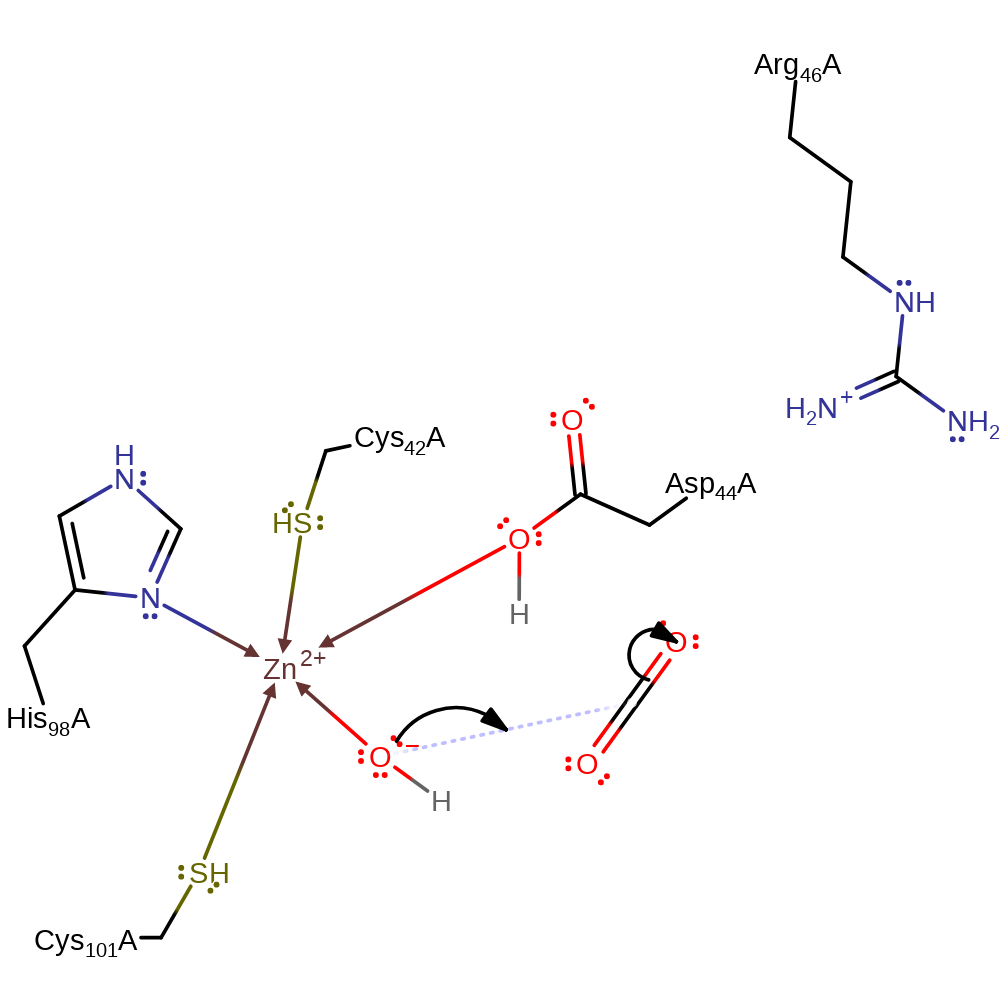
Step 2. The hydroxide is now activated for nucleophilic attack on the carbon dioxide.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys42A | metal ligand |
| Asp44A | metal ligand |
| His98A | metal ligand |
| Cys101A | metal ligand |
Chemical Components
ingold: bimolecular nucleophilic addition, overall reactant used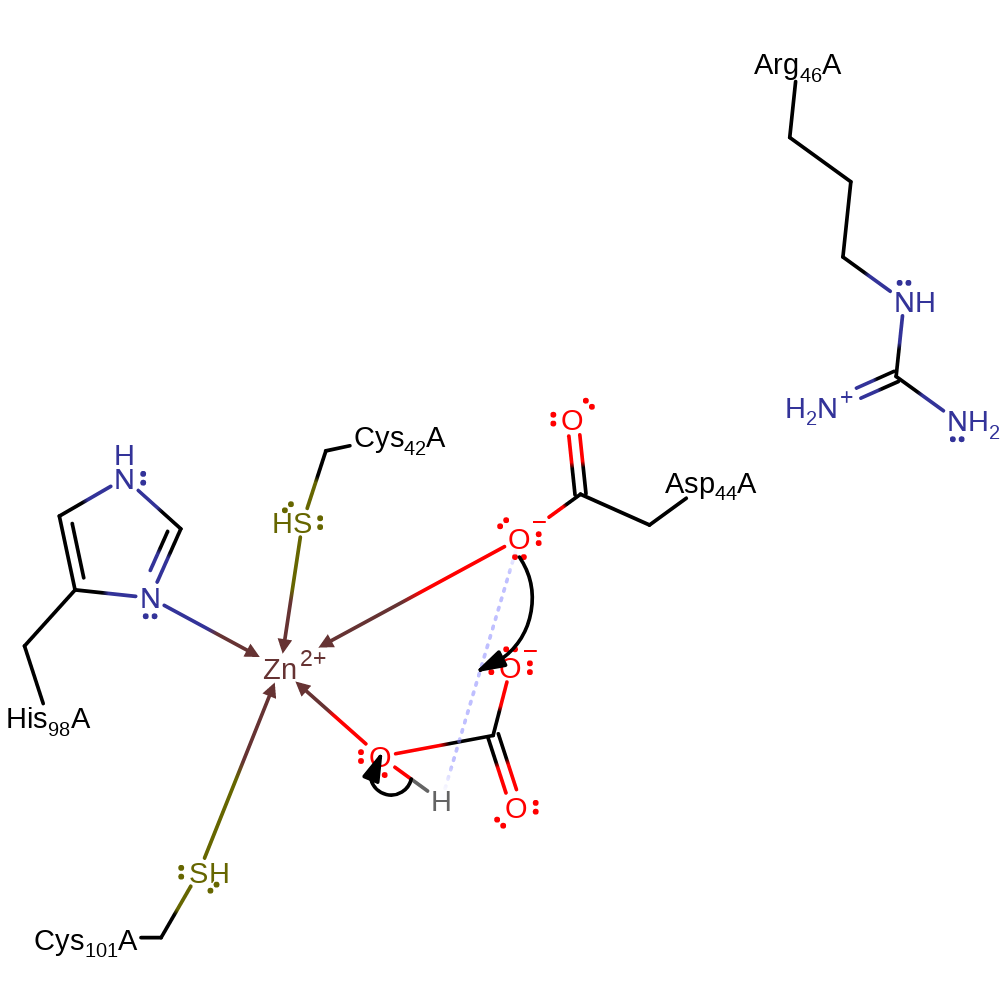
Step 3. Deprotonation is catalyzed by Asp 44 transferring the proton to the solvent, allowing the product to leave the active site.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys42A | metal ligand |
| Asp44A | metal ligand |
| His98A | metal ligand |
| Cys101A | metal ligand |
| Arg46A | increase basicity |
| Arg46A | electrostatic stabiliser |
| Asp44A | proton acceptor |




 Download:
Download: