Alpha-amylase
Alpha-amylase catalyses the hydrolysis of internal alpha-glucosidic linkages in starch and other related oligo- and polysaccharides. These enzymes are widespread among the higher plants, animals and micro-organisms.
Cereal alpha-amylases play an important role in the production of beer and other alcoholic beverages.
In humans, alpha-amylase is present in salivary and pancreatic secretions. Human pancreatic alpha-amylase (HPA) is a 496 amino acid single polypeptide chain which binds to essential calcium and chloride ions, and is responsible for the hydrolysis of the partially digested starch (smaller oligosaccharides) reaching the gut, into glucose. Inhibition of HPA provides an effective target for the treatment of diabetes.
Reference Protein and Structure
- Sequence
-
P04063
 (3.2.1.1)
(3.2.1.1)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Hordeum vulgare (Barley)

- PDB
-
1amy
- CRYSTAL AND MOLECULAR STRUCTURE OF BARLEY ALPHA-AMYLASE
(2.8 Å)



- Catalytic CATH Domains
-
3.20.20.80
 (see all for 1amy)
(see all for 1amy)
- Cofactors
- Calcium(2+) (1)
Enzyme Reaction (EC:3.2.1.1)
Enzyme Mechanism
Introduction
This reaction occurs via a double displacement mechanism involving the formation and hydrolysis of a covalent beta-glycosyl enzyme intermediate.
- Formation of the intermediate involves attack at the sugar anomeric centre by a nucleophilic Asp 179 side chain. This is assisted by general acid catalysis of Glu 204 and Asp 289.
- The covalent glycosyl intermediate undergoes general base catalysed hydrolysis via attack of nucleophilic water at the anomeric centre, again catalysed by Glu 204 and Asp 289. (Glu 204 and Asp 289 side chains deprotonate the water, activating it towards nucleophilic attack). These residues also form hydrogenbonds to the water molecule.
- This leads to an oxocarbenium ion-like transition state, which goes on to form the deglycosylated product.
Catalytic Residues Roles
| UniProt | PDB* (1amy) | ||
| Asp203 | Asp179A | Acts as the catalytic nucleophile, attacking the sugar anomeric centre. | covalent catalysis |
| Asp313 | Asp289A | Acts as a general acid/base. Nucleophilic attack at the sugar anomeric centre is assisted by acid/base catalysis of the anomeric OR' with the Asp side chain. Deprotonation of the nucleophilic water activates it towards attack of the anomeric centre.H bonding to the water molecule also occurs. Asp 300 also aids the deformation of the substrate and enhances sugar electrophilicity at the anomeric centre. | hydrogen radical acceptor, proton shuttle (general acid/base), electrostatic stabiliser |
| Glu228 | Glu204A | Acts as a general acid/base. Nucleophilic attack at the sugar anomeric centre is assisted by acid/base catalysis of the anomeric OR' with the Glu side chain. Deprotonation of the nucleophilic water activates it towards attack of the anomeric centre.H bonding to the water molecule also occurs. | hydrogen radical acceptor, proton shuttle (general acid/base) |
Chemical Components
intermediate formation, bimolecular nucleophilic addition, enzyme-substrate complex formation, proton transfer, hydrolysis, overall product formed, enzyme-substrate complex cleavageReferences
- Rydberg EH et al. (2002), Biochemistry, 41, 4492-4502. Mechanistic analyses of catalysis in human pancreatic alpha-amylase: detailed kinetic and structural studies of mutants of three conserved carboxylic acids. DOI:10.2210/pdb1kbk/pdb. PMID:11914097.
- Pinto GP et al. (2015), J Chem Theory Comput, 11, 2508-2516. Establishing the catalytic mechanism of human pancreatic α-amylase with QM/MM methods. DOI:10.1021/acs.jctc.5b00222. PMID:26575550.
- Yoon SH et al. (2007), Carbohydr Res, 342, 55-64. Formation of covalent β-linked carbohydrate–enzyme intermediates during the reactions catalyzed by α-amylases. DOI:10.1016/j.carres.2006.10.028. PMID:17123489.
- Brayer GD et al. (2000), Biochemistry, 39, 4778-4791. Subsite Mapping of the Human Pancreatic α-Amylase Active Site through Structural, Kinetic, and Mutagenesis Techniques†,‡. DOI:10.1021/bi9921182. PMID:10769135.
- Kadziola A et al. (1998), J Mol Biol, 278, 205-217. Molecular structure of a barley α-amylase-inhibitor complex: implications for starch binding and catalysis. DOI:10.1006/jmbi.1998.1683. PMID:9571044.
- Davies G et al. (1995), Structure, 3, 853-859. Structures and mechanisms of glycosyl hydrolases. DOI:10.1016/s0969-2126(01)00220-9. PMID:8535779.
- Kadziola A et al. (1994), J Mol Biol, 239, 104-121. Crystal and molecular structure of barley alpha-amylase. DOI:10.2210/pdb1amy/pdb. PMID:8196040.
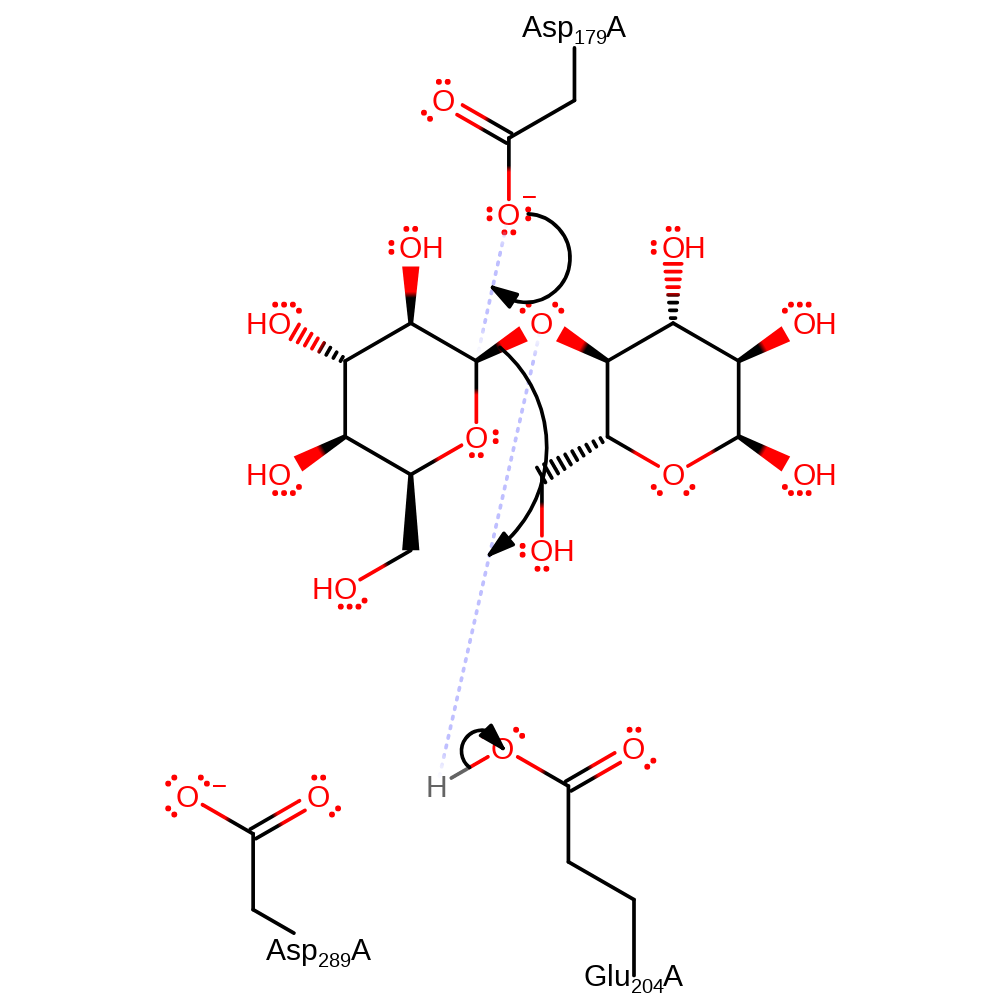
Step 1. Nucleophilic attack at the sugar anomeric centre by Asp179 side chain. This is assisted by general acid catalysis by Glu204 and Asp289. H atoms omitted for clarity.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp289A | electrostatic stabiliser |
| Asp179A | covalent catalysis |
| Glu204A | proton shuttle (general acid/base) |
| Asp289A | proton shuttle (general acid/base) |
Chemical Components
intermediate formation, ingold: bimolecular nucleophilic addition, enzyme-substrate complex formation, proton transfer, hydrolysis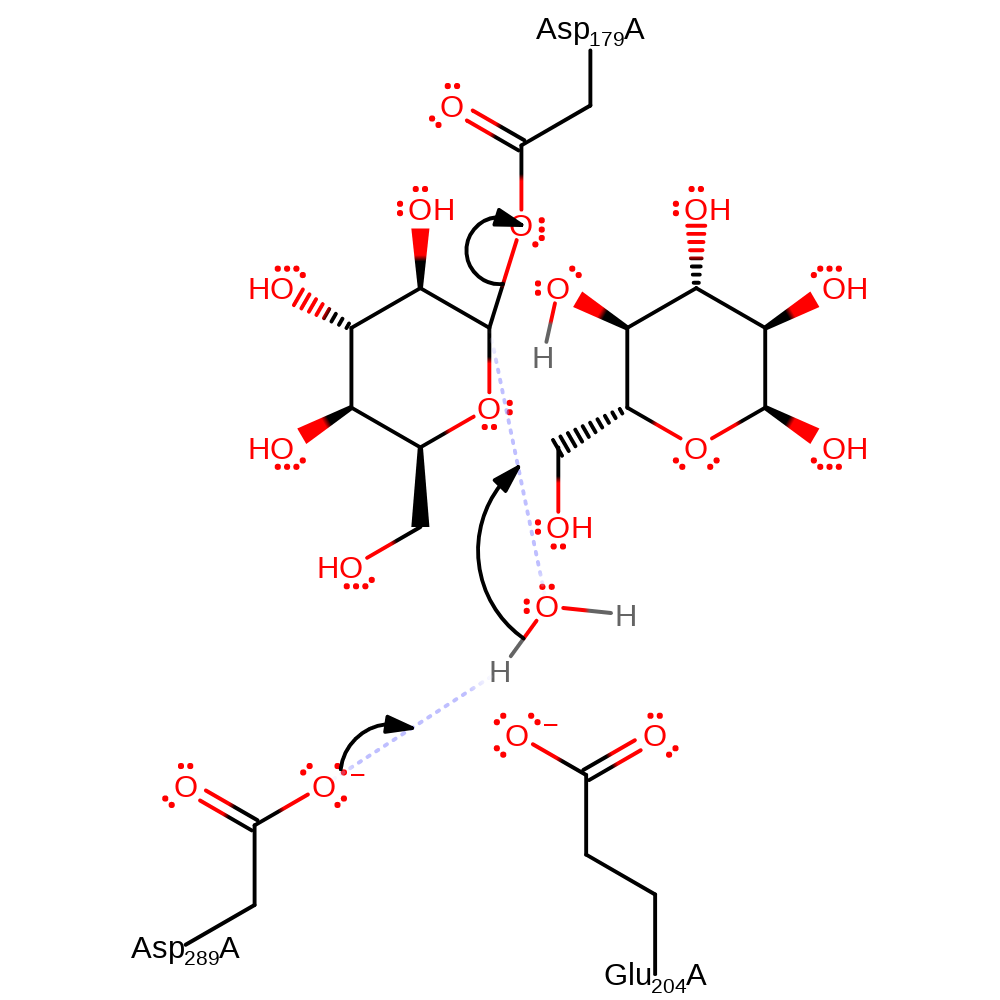
Step 2. The covalent glycosyl intermediate undergoes general base catalysed hydrolysis via attack of nucleophilic water at the anomeric centre, again catalysed by Glu204 and Asp289. These residues also form hydrogen bonds to the molecule. This leads to a oxocarbenium ion-like transition state, which forms the deglycosylated product.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp179A | covalent catalysis |
| Glu204A | hydrogen radical acceptor |
| Asp289A | hydrogen radical acceptor |
| Glu204A | proton shuttle (general acid/base) |
| Asp289A | proton shuttle (general acid/base) |



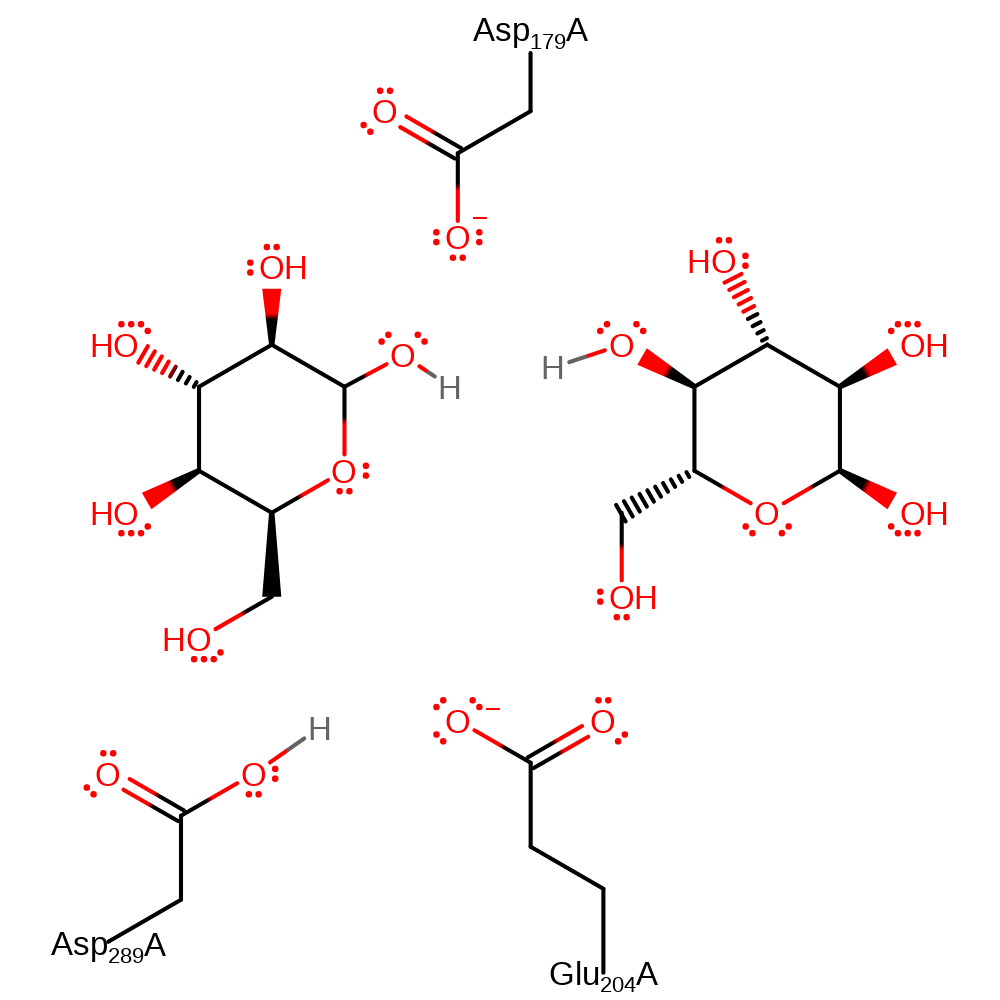 Download:
Download: