Lysine 2,3-aminomutase
Lysine 2,3-aminomutase (LAM) is a member of the Radical SAM superfamily and is involved in the metabolism of lysine. The superfamily is characterised by the presence of a CxxxCxxC motif which binds a [4Fe-4S] cluster, leaving one of the iron ions unsatisfied. It is often found in anaerobic bacteria that utilise (S)-lysine for growth as a source of nitrogen and/or carbon. Biosynthetically, the (S)-beta-lysine produced is incorporated into antibiotics that contain beta-aminoacyl substituents. Whilst most LAM enzymes produce the (S)-beta-lysine, some produce the R form, such as the Escherichia coli gene yjeK which encodes for a variant of the classical LAM. Whilst zinc is required for enzyme activity,the Zn ion is structural, rather than a cofactor and is required for maintaining the oligomeric state of the biological unit.
Reference Protein and Structure
- Sequence
-
Q9XBQ8
 (5.4.3.2)
(5.4.3.2)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Clostridium subterminale (Bacteria)

- PDB
-
2a5h
- 2.1 Angstrom X-ray crystal structure of lysine-2,3-aminomutase from Clostridium subterminale SB4, with Michaelis analog (L-alpha-lysine external aldimine form of pyridoxal-5'-phosphate).
(2.1 Å)



- Catalytic CATH Domains
-
3.20.20.70
 (see all for 2a5h)
(see all for 2a5h)
- Cofactors
- S-adenosyl-l-methionine (1), Tetra-mu3-sulfido-tetrairon (1), Pyridoxal 5'-phosphate(2-) (1), Water (1)
Enzyme Reaction (EC:5.4.3.2)
Enzyme Mechanism
Introduction
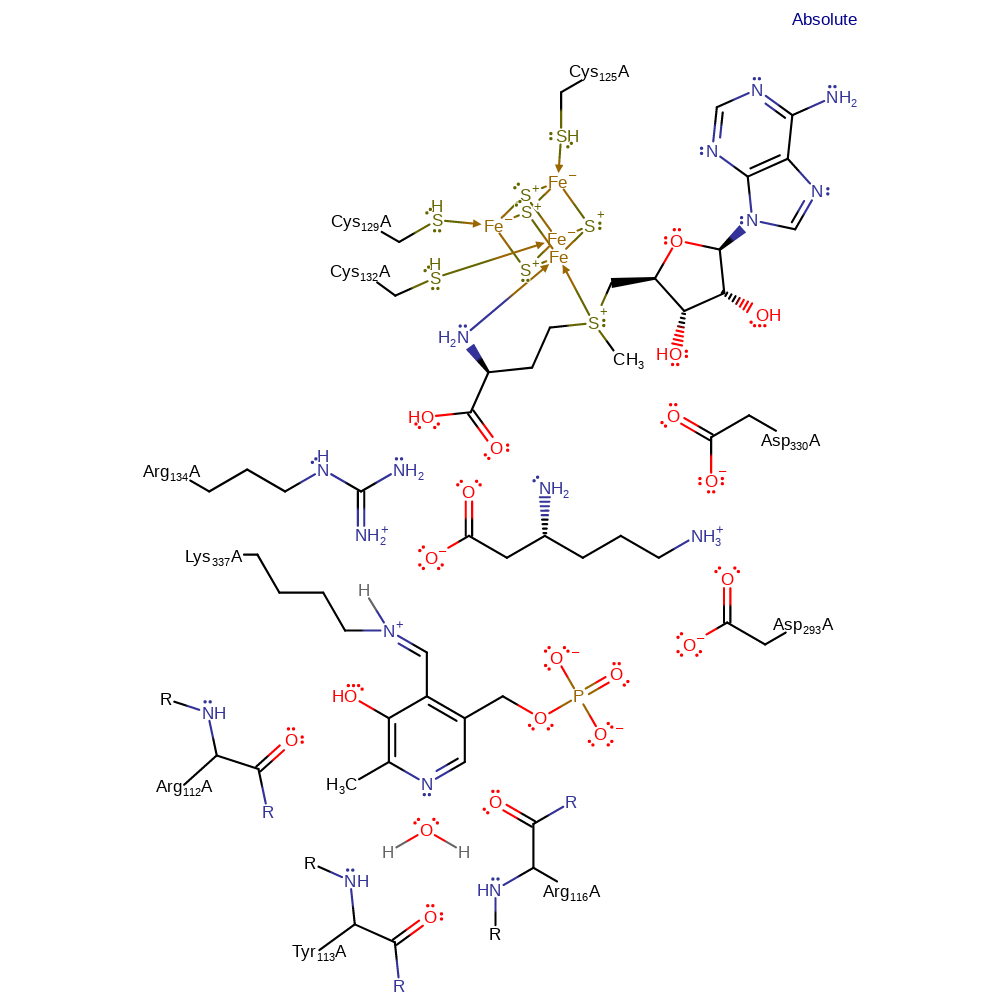
LAM has a strict requirement for S-adenosyl-L-methionine, a [4Fe-4S]+ cluster and pyridoxal 5'-phosphate (PLP), as well as anaerobic conditions. The PLP is bound in the active site in a unique mode, in which the pyrimidine nitrogen is neutral and unprotonated being hydrogen bonded to a strictly conserved water molecule, which is in turn held in place by the main chain portions of R116, R112 and Y113.
Catalytic Residues Roles
| UniProt | PDB* (2a5h) | ||
| Arg112 (main-N), Tyr113 (main-N), Arg116 (main-C) | Arg112A (main-N), Tyr113A (main-N), Arg116A (main-C) | Hold the strictly conserved water molecule in place by the main chain portions of R116, R112 and Y113. | hydrogen bond donor, steric role, electrostatic stabiliser |
| Arg134 | Arg134A | Binds the carboxylate group of the substrate, helps maintain the substrate in the correct orientation for the reaction to occur. | hydrogen bond donor, steric role, electrostatic stabiliser |
| Lys337 | Lys337A | Bound to the PLP cofactor in the ground state of the enzyme. Acts via covalent catalysis and as a general acid/base. | covalently attached, hydrogen bond donor, nucleophile, proton acceptor, proton donor, nucleofuge, electron pair acceptor, electron pair donor |
| Asp293, Asp330 | Asp293A, Asp330A | Bind the ammonium group of the lysine substrate, holding it in place to ensure the correct reaction occurs. | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Cys132, Cys129, Cys125 | Cys132A, Cys129A, Cys125A | Bind the [4Fe-4S]-AdoMet cluster. | activator, metal ligand |
Chemical Components
intramolecular homolytic elimination, electron transfer, intermediate formation, cofactor used, bimolecular nucleophilic addition, proton transfer, intramolecular elimination, hydrogen transfer, intramolecular homolytic substitution, cyclisation, atom stereo change, decyclisation, native state of cofactor regenerated, bimolecular homolytic addition, native state of enzyme regenerated, intermediate terminatedReferences
- Lepore BW et al. (2005), Proc Natl Acad Sci U S A, 102, 13819-13824. The x-ray crystal structure of lysine-2,3-aminomutase from Clostridium subterminale. DOI:10.1073/pnas.0505726102. PMID:16166264.
- Frey PA et al. (2011), Biochim Biophys Acta, 1814, 1548-1557. Pyridoxal-5′-phosphate as the catalyst for radical isomerization in reactions of PLP-dependent aminomutases. DOI:10.1016/j.bbapap.2011.03.005. PMID:21435400.
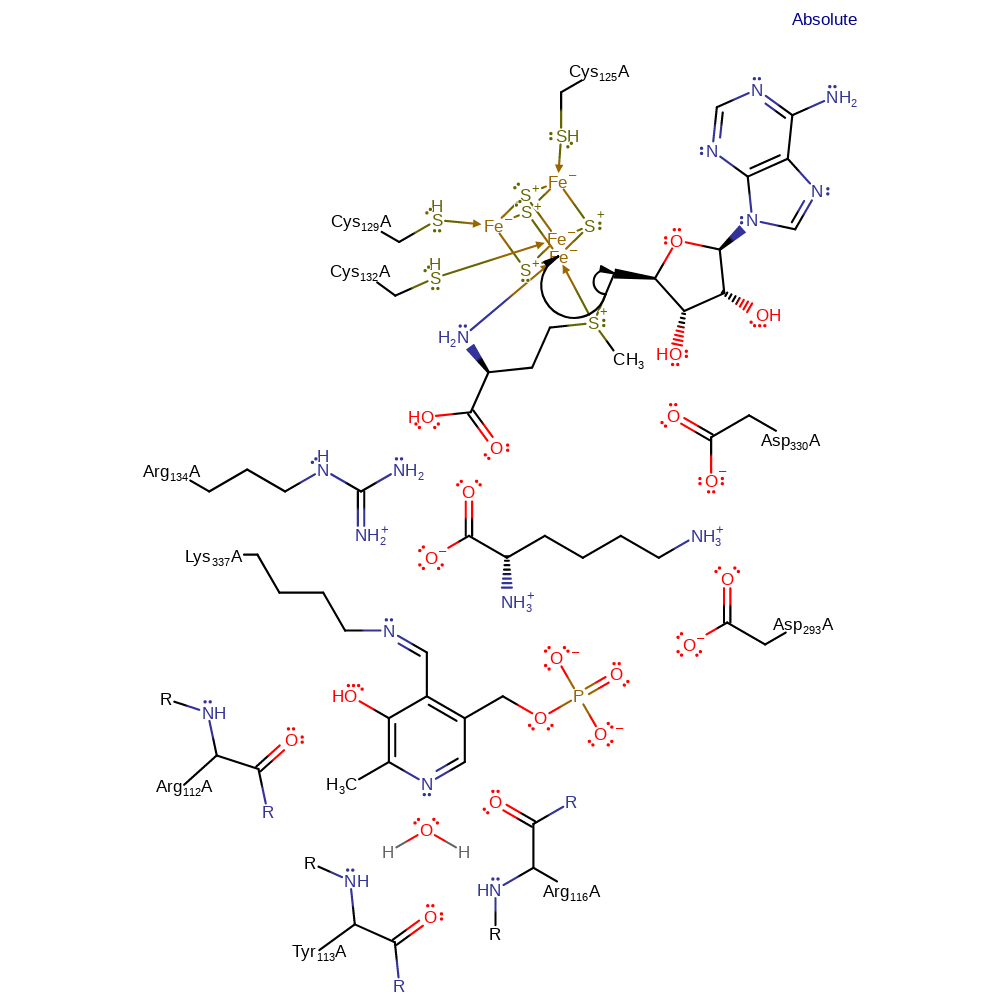
Step 1. Activation step in which the 5'C-S bond in SAM is reductively cleaved via the iron-sulfur cluster, generating the catalytic 5'-deoxyadenosyl radical.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys125A | metal ligand, activator |
| Cys129A | metal ligand, activator |
| Cys132A | metal ligand, activator |
| Arg116A (main-C) | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg112A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Tyr113A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Asp293A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg134A | steric role, hydrogen bond donor, electrostatic stabiliser |
| Asp330A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Lys337A | covalently attached |
Chemical Components
ingold: intramolecular homolytic elimination, electron transfer, intermediate formation, cofactor used
Step 2. The first transaldimination step in which the substrate lysine attacks the PLP in a nucleophilic addition reaction.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys125A | metal ligand |
| Cys129A | metal ligand |
| Cys132A | metal ligand |
| Arg116A (main-C) | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg112A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Tyr113A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Asp293A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg134A | steric role, hydrogen bond donor, electrostatic stabiliser |
| Asp330A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Lys337A | covalently attached |
| Lys337A | proton acceptor, electron pair acceptor |
Chemical Components
ingold: bimolecular nucleophilic addition, proton transfer, intermediate formation, cofactor used
Step 3. The final step in the transaldimination reaction, generating the PLP-Lys intermediate and free Lys337 in an elimination reaction.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys125A | metal ligand |
| Cys129A | metal ligand |
| Cys132A | metal ligand |
| Arg116A (main-C) | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg112A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Tyr113A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Asp293A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg134A | steric role, hydrogen bond donor, electrostatic stabiliser |
| Asp330A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Lys337A | proton acceptor, nucleofuge |
Chemical Components
ingold: intramolecular elimination, intermediate formation
Step 4. The 5'-deoxyadenosenyl radical abstracts a hydrogen from the PLP-Lys intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys125A | metal ligand |
| Cys129A | metal ligand |
| Cys132A | metal ligand |
| Arg116A (main-C) | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg112A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Tyr113A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Asp293A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg134A | steric role, hydrogen bond donor, electrostatic stabiliser |
| Asp330A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Lys337A | hydrogen bond donor |
Chemical Components
hydrogen transfer, intermediate formation
Step 5. The radical product undergoes intramolecular rearrangement to produce a cyclic intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys125A | metal ligand |
| Cys129A | metal ligand |
| Cys132A | metal ligand |
| Arg116A (main-C) | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg112A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Tyr113A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Asp293A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg134A | steric role, hydrogen bond donor, electrostatic stabiliser |
| Asp330A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Lys337A | hydrogen bond donor |
Chemical Components
ingold: intramolecular homolytic substitution, intermediate formation, cyclisation, atom stereo changeCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys125A | metal ligand |
| Cys129A | metal ligand |
| Cys132A | metal ligand |
| Arg116A (main-C) | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg112A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Tyr113A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Asp293A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg134A | steric role, hydrogen bond donor, electrostatic stabiliser |
| Asp330A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Lys337A | hydrogen bond donor |
Chemical Components
ingold: intramolecular homolytic elimination, intermediate formation, decyclisation, atom stereo change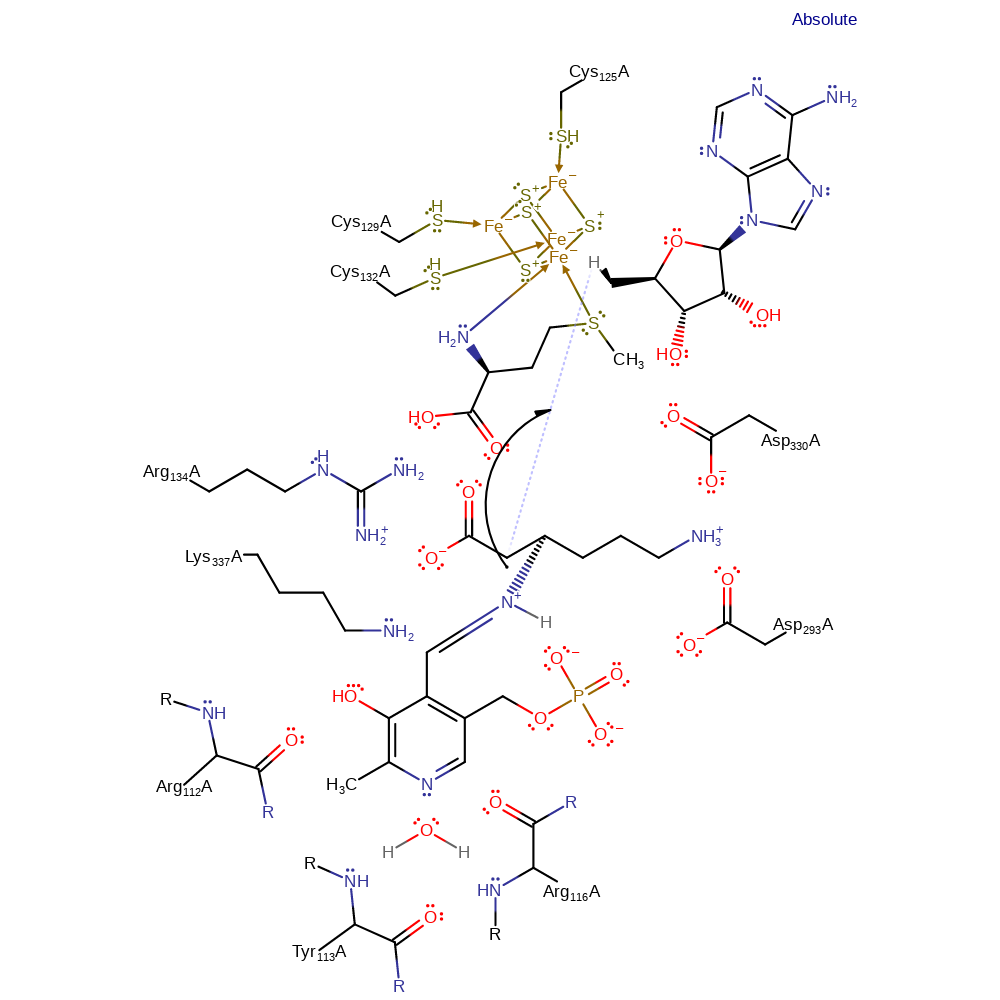
Step 7. The radical intermediate abstractes a hydrogen from the deoxyadenosyl intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys125A | metal ligand |
| Cys129A | metal ligand |
| Cys132A | metal ligand |
| Arg116A (main-C) | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg112A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Tyr113A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Asp293A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg134A | steric role, hydrogen bond donor, electrostatic stabiliser |
| Asp330A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Lys337A | hydrogen bond donor |
Chemical Components
hydrogen transfer, intermediate formation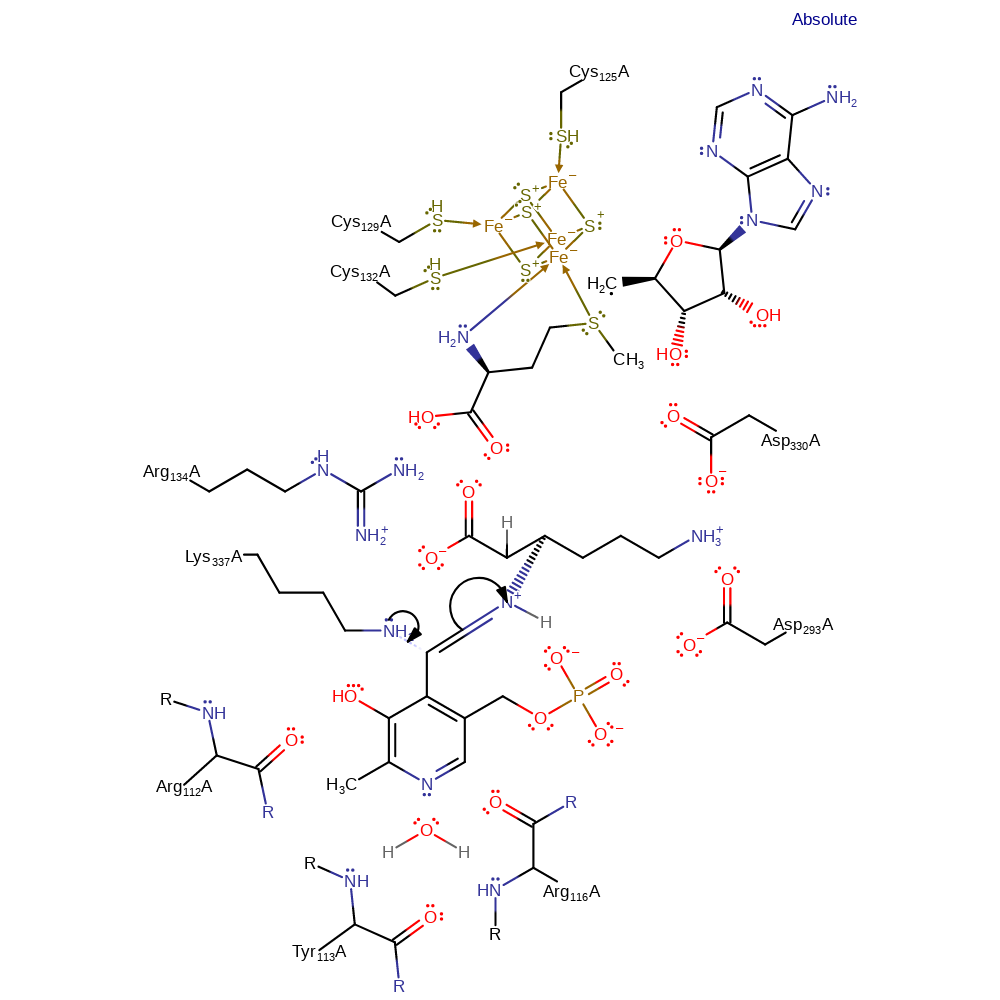
Step 8. The first reverse-transaldimination step in which the protein lysine attacks the PLP in a nucleophilic addition reaction.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys125A | metal ligand |
| Cys129A | metal ligand |
| Cys132A | metal ligand |
| Arg116A (main-C) | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg112A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Tyr113A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Asp293A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg134A | steric role, hydrogen bond donor, electrostatic stabiliser |
| Asp330A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Lys337A | nucleophile |
Chemical Components
ingold: bimolecular nucleophilic addition, proton transfer, intermediate formation, native state of cofactor regenerated
Step 9. The final step in the transaldimination reaction, re-generating the PLP-(Enz)Lys and bela-lysine product in an elimination reaction.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys125A | metal ligand |
| Cys129A | metal ligand |
| Cys132A | metal ligand |
| Arg116A (main-C) | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg112A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Tyr113A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Asp293A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg134A | steric role, hydrogen bond donor, electrostatic stabiliser |
| Asp330A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Lys337A | covalently attached |
| Lys337A | proton donor, electron pair donor |
Chemical Components
ingold: intramolecular elimination, intermediate formation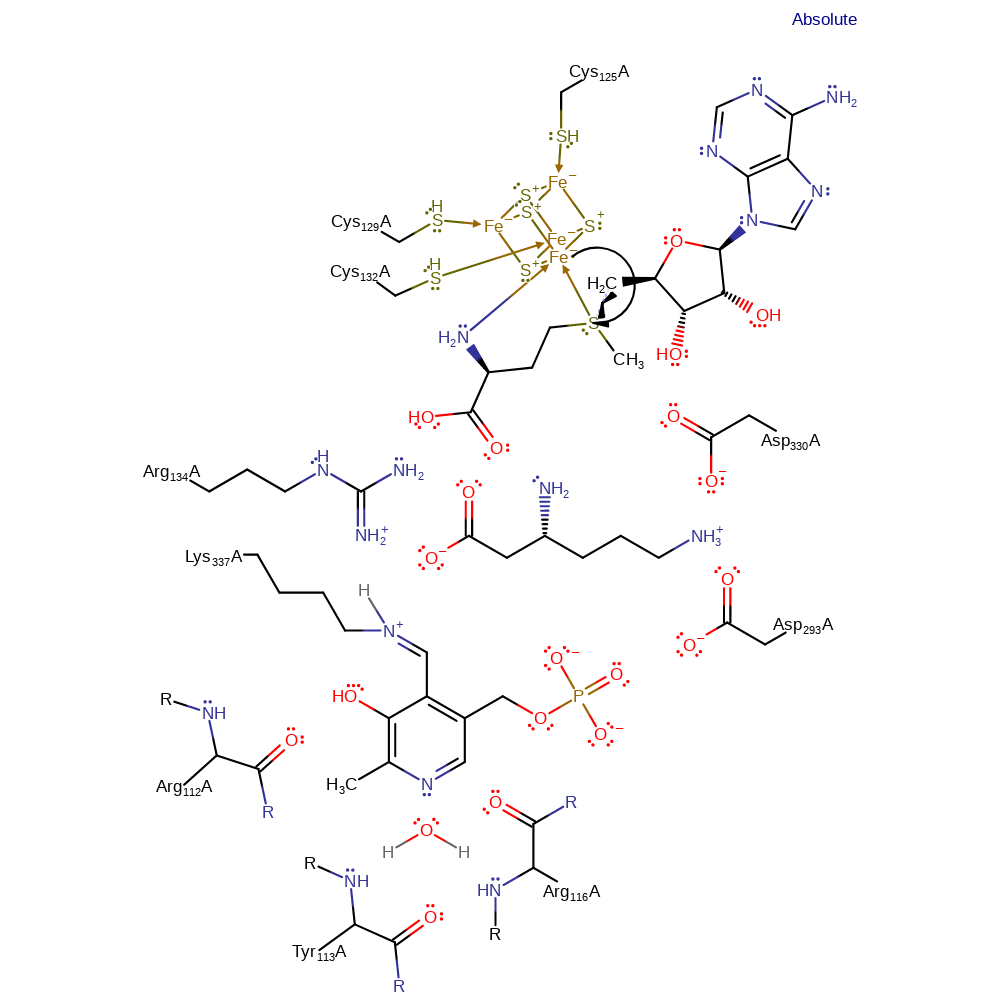
Step 10. The 5'-deoxyadenosyl is quenched, regenerating the SAM and iron-sulfur cluster in the reverse of the first step.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys125A | metal ligand, activator |
| Cys129A | metal ligand, activator |
| Cys132A | metal ligand, activator |
| Arg116A (main-C) | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg112A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Tyr113A (main-N) | hydrogen bond donor, steric role, electrostatic stabiliser |
| Asp293A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Arg134A | steric role, hydrogen bond donor, electrostatic stabiliser |
| Asp330A | hydrogen bond acceptor, steric role, electrostatic stabiliser |
| Lys337A | covalently attached |



 Download:
Download: