Phycoerythrobilin synthase
Plays a role in phycoerythrobilin biosynthesis, the red pigment chromophore photosynthetically active biliproteins of a cyanophage infecting oceanic cyanobacteria of the Prochlorococcus genus. It uses a four electron reduction to carry out the reactions catalyses by EC 1.3.7.2 (15,16-dihydrobiliverdin:ferredoxin oxidoreductase) and EC 1.3.7.3 (phycoerythrobilin:ferredoxin oxidoreductase). 15,16-Dihydrobiliverdin is formed as a bound intermediate. Free 15,16-dihydrobiliverdin can also act as a substrate to form phycoerythrobilin.
Reference Protein and Structure
- Sequence
-
Q58MU6
 (1.3.7.6)
(1.3.7.6)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Prochlorococcus phage P-SSM2 (Virus)

- PDB
-
2vck
- Structure of Phycoerythrobilin Synthase PebS from the Cyanophage P-SSM2 in Complex with the bound Substrate Biliverdin IXa
(1.8 Å)



- Catalytic CATH Domains
-
3.40.1500.20
 (see all for 2vck)
(see all for 2vck)
Enzyme Mechanism
- Summary
- Step 1
- Step 2
- Step 3
- Step 4
- Step 5
- Step 6
- Step 7
- Step 8
- Step 9
- Step 10
- Step 11
- Products
- All Steps
Introduction
Spectroscopic studies have shown the ring to adopt a number of protonation states throughout the reaction [PMID:21050180]. The pyrrole ring amide functionality of the fourth ring undergoes tautomerisation. Simultaneously, reduced ferredoxin delivers one electron to the biliverdin ring while a solvent molecule donates a proton. This generates a neutral radical species. The single electron oxidised ferredoxin donates a second electron in conjunction with Asp105A donating a proton to the C15 position of biliverdin. Asp105A initiates tautomerisation in the intermediate. Asp105A is deprotonated in a stereospecific manner, completing the initial tautomerisation. The pyrrole ring now undergoes a spontaneous tautomerisation. A second ferredoxin donates an electron to the first pyrrole ring, initiating deprotonation of Asp206. Asp105 activates the radical intermediate towards tautomersiation. Asp105A acts as a stereospecific general acid at the C2 position of the radical intermediate. The final electron transfer occurs with concurrent deprotonation of a solvent molecule. Reprotonation of the two catalytic aspartate residues regenerates the active site.
Catalytic Residues Roles
| UniProt | PDB* (2vck) | ||
| Asp206, Asp105 | Asp206A, Asp105A | Acts as a general acid/base. | hydrogen bond acceptor, hydrogen bond donor, proton acceptor, proton donor |
Chemical Components
tautomerisation (not keto-enol), intermediate formation, overall reactant used, electron transfer, proton transfer, overall product formed, assisted tautomerisation (not keto-enol), assisted keto-enol tautomerisation, native state of enzyme regenerated, inferred reaction stepReferences
- Busch AW et al. (2011), Biochem J, 433, 469-476. Radical mechanism of cyanophage phycoerythrobilin synthase (PebS). DOI:10.1042/bj20101642. PMID:21050180.
- Hagiwara Y et al. (2010), J Biol Chem, 285, 1000-1007. Structural Insights into Vinyl Reduction Regiospecificity of Phycocyanobilin:Ferredoxin Oxidoreductase (PcyA). DOI:10.1074/jbc.m109.055632. PMID:19887371.
- Chiu FY et al. (2010), J Biol Chem, 285, 5056-5065. Electrostatic Interaction of Phytochromobilin Synthase and Ferredoxin for Biosynthesis of Phytochrome Chromophore. DOI:10.1074/jbc.m109.075747. PMID:19996315.
- Dammeyer T et al. (2008), J Biol Chem, 283, 27547-27554. Phycoerythrobilin Synthase (PebS) of a Marine Virus: CRYSTAL STRUCTURES OF THE BILIVERDIN COMPLEX AND THE SUBSTRATE-FREE FORM. DOI:10.1074/jbc.m803765200. PMID:18662988.
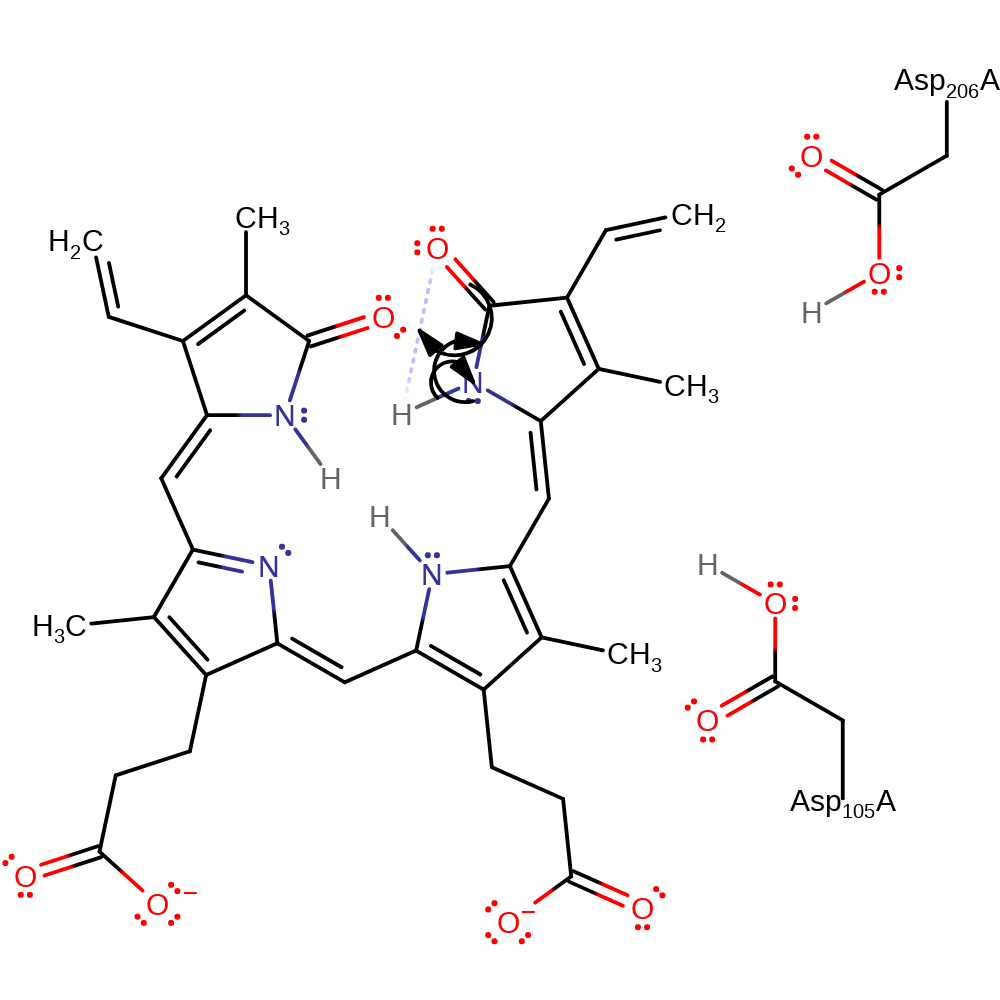
Step 1. The pyrrole ring amide functionality of the fourth ring undergoes tautomerisation.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp105A | hydrogen bond donor |
| Asp206A | hydrogen bond donor |
Chemical Components
tautomerisation (not keto-enol), intermediate formation, overall reactant used
Step 2. Simultaneously, reduced ferredoxin delivers one electron to the biliverdin ring while a solvent molecule donates a proton. This generates a neutral radical species. The presence of a conserved region of positively charged residues on the protein surface binds the electron transfer protein ferredoxin. The ionisable carboxylate side chains on the biliverdin substrate are proposed to be involved in mediating electron transfer between the ferredoxin binding site and the internal lactam rings [PMID:19996315].
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp206A | hydrogen bond donor |
| Asp105A | hydrogen bond donor |
Chemical Components
electron transfer, proton transfer, intermediate formation, overall reactant used
Step 3. The single electron oxidised ferredoxin donates a second electron in conjunction with Asp105A donating a proton to the C15 position of biliverdin.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp105A | hydrogen bond donor |
| Asp206A | hydrogen bond donor |
| Asp105A | proton donor |
Chemical Components
electron transfer, proton transfer, intermediate formation, overall product formedCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp206A | hydrogen bond donor |
| Asp105A | hydrogen bond acceptor |
| Asp105A | proton acceptor |
Chemical Components
assisted tautomerisation (not keto-enol), proton transfer, intermediate formation
Step 5. Asp105A is deprotonated in a stereospecific manner, completing the initial tautomerisation. The catalytic aspartate residues are positioned as to act as stereospecific general bases towards the reaction intermediates [PMID:19887371, PMID:21050180].
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp105A | hydrogen bond donor, steric role |
| Asp206A | hydrogen bond donor |
| Asp105A | proton donor |
Chemical Components
proton transfer, intermediate formationCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp105A | hydrogen bond acceptor |
| Asp206A | hydrogen bond donor |
Chemical Components
tautomerisation (not keto-enol), intermediate formation
Step 7. A second ferredoxin donates an electron to the first pyrrole ring, initiating deprotonation of Asp206.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp206A | hydrogen bond donor |
| Asp105A | hydrogen bond acceptor |
| Asp206A | proton donor |
Chemical Components
electron transfer, proton transfer, overall reactant used, intermediate formation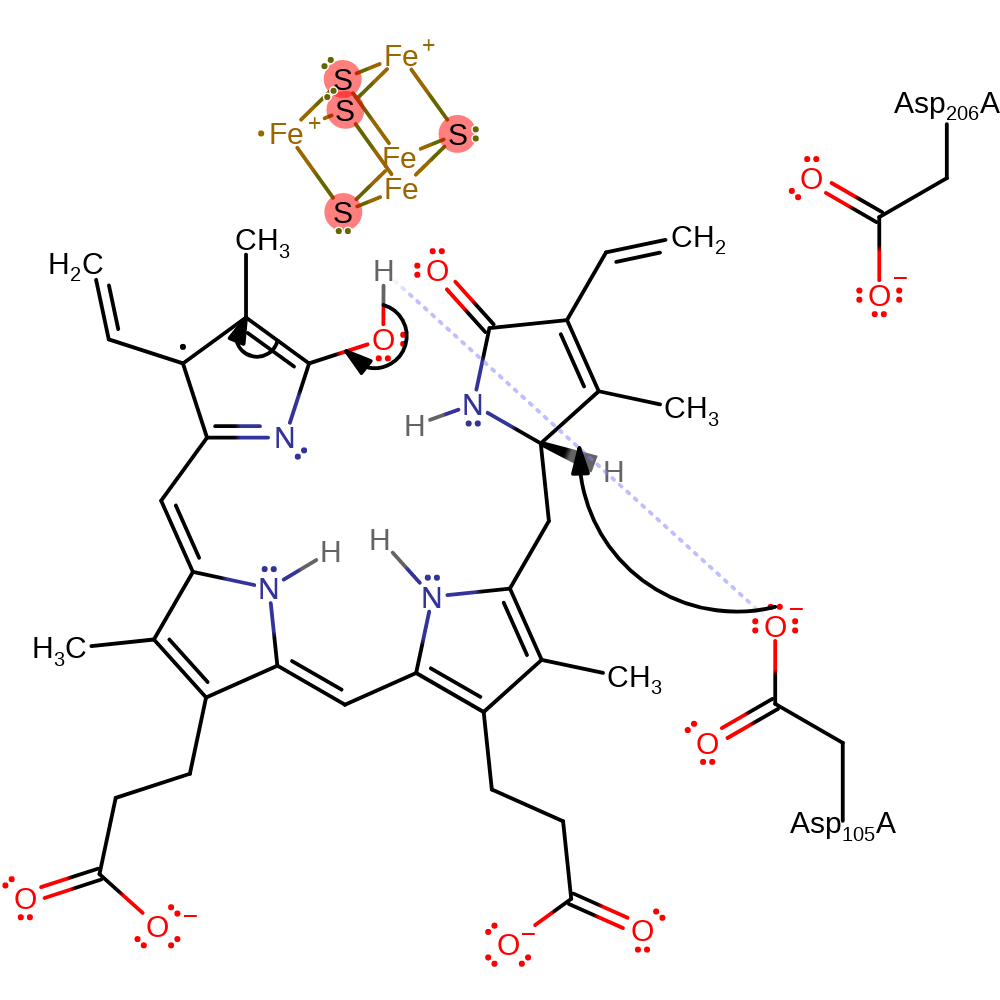
Step 8. Asp105 activates the radical intermediate towards tautomersiation.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp105A | hydrogen bond acceptor |
| Asp206A | hydrogen bond acceptor |
| Asp105A | proton acceptor |
Chemical Components
proton transfer, assisted keto-enol tautomerisation, intermediate formation
Step 9. Asp105A acts as a stereospecific general acid at the C2 position of the radical intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp105A | hydrogen bond donor, steric role |
| Asp206A | hydrogen bond acceptor |
| Asp105A | proton donor |
Chemical Components
proton transfer
Step 10. The final electron transfer occurs with concurrent deprotonation of a solvent molecule.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp105A | hydrogen bond acceptor |
| Asp206A | hydrogen bond acceptor |
Chemical Components
proton transfer, electron transfer, overall product formed, overall reactant used
Step 11. Reprotonation of the two catalytic aspartate residues regenerates the active site.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp105A | hydrogen bond acceptor |
| Asp206A | hydrogen bond acceptor, proton acceptor |
| Asp105A | proton acceptor |







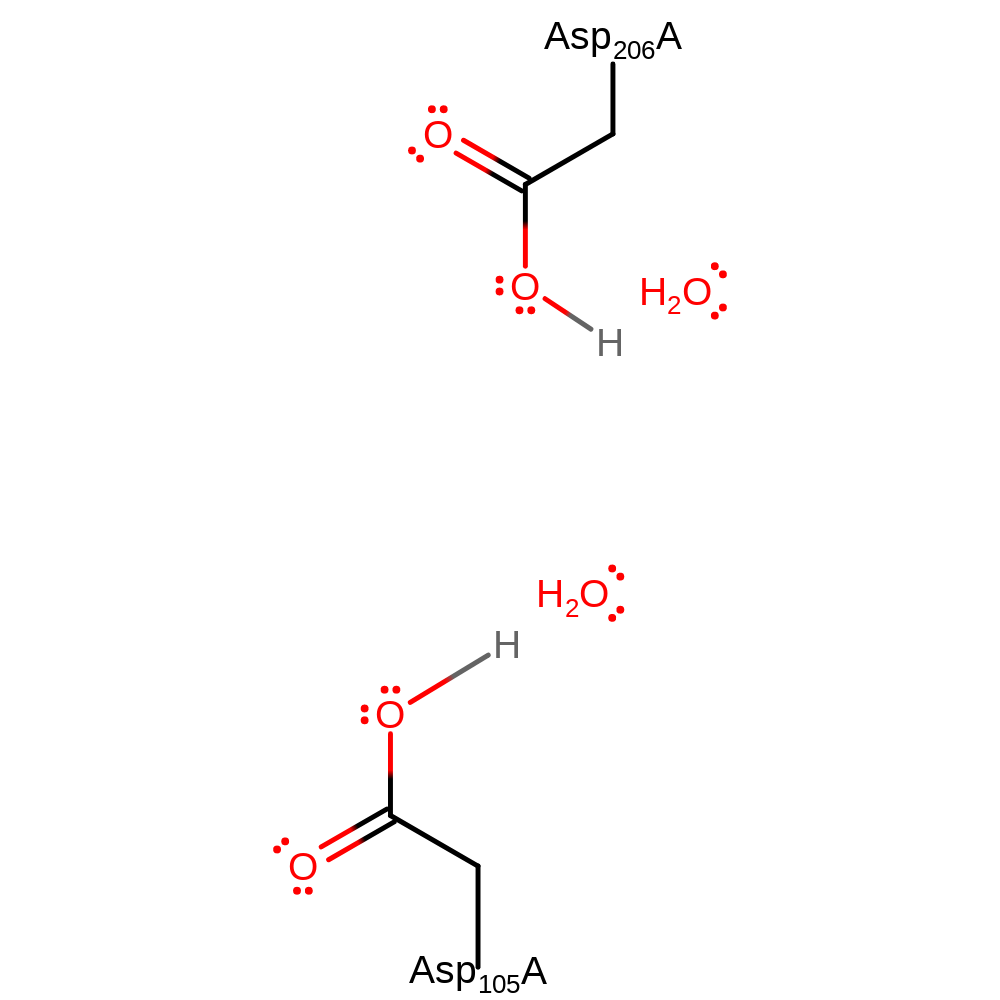 Download:
Download: