Peroxidase
Horseradish peroxidase C (HRPC) is the most studied member of the class III peroxidases. These enzymes occur in higher plants and catalyse the oxidation of phenolic compounds to phenol radicals using hydrogen peroxide which is reduced to water. Since plant peroxidases generally have broad substrate specificity and produce highly reactive radical products which can participate in non-enzymatic reactions, the biological functions of these enzymes have been difficult to verify. They have been proposed to have roles in lignin production and cell wall formation, for example by catalysing the formation of monolignol radicals which subsequently polymerise to lignin, and by catalysing cell wall cross-linking reactions involving ferulic acid.
Reference Protein and Structure
- Sequence
-
P00433
 (1.11.1.7)
(1.11.1.7)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Armoracia rusticana (Horseradish)

- PDB
-
7atj
- RECOMBINANT HORSERADISH PEROXIDASE C1A COMPLEX WITH CYANIDE AND FERULIC ACID
(1.47 Å)



- Catalytic CATH Domains
-
1.10.520.10
 1.10.420.10
1.10.420.10  (see all for 7atj)
(see all for 7atj)
- Cofactors
- Heme b (1) Metal MACiE
Enzyme Reaction (EC:1.11.1.7)
Enzyme Mechanism
Introduction
The reaction occurs in three steps:
The enzyme is first oxidised by hydrogen peroxide and is then reduced in two sequential one-electron transfer steps from reducing substrates (typically small molecule phenol derivatives). The mechanism of the first step is believed to be common to all haem peroxidases. HOOH is deprotonated by His 42 to produce singly ionised Fe(III)-coordinated peroxide. The O-O bond of the coordinated peroxide is now heterolytically cleaved to generate an Fe(IV)=O species and a hydroxide ion, which is protonated by His 42. Arg 38 is though to promote the reaction by stabilising negative charge in the transient Fe(III)-OOH intermediate and associated transition states.
In the second step, a reducing phenol substrate binds to the active site and transfers an electron to haem and a proton to His 42. The proton transfer is thought to occur via an active site water molecule, and is proposed to be assisted by Arg 38 which forms a hydrogen bond to the substrate phenolic oxygen.
The product radical now dissociates from the active site and a second reducing phenol substrate is bound. This second reducing substrate transfers an electron to the haem, and a proton (via an active site water) to the Fe-coordinated O atom. This is accompanied by proton transfer from His 42 to the Fe-coordinated oxygen, so that the overall result is formation of a water molecule and regeneration of the resting state haem Fe(III).
Catalytic Residues Roles
| UniProt | PDB* (7atj) | ||
| Arg68 | Arg38A | Stabilises negative charge in the transient Fe(III)-OOH intermediate and associated transition states. Assists proton transfer from the phenol substrates to either His 42 or the ferryl oxygen. | hydrogen bond acceptor, hydrogen bond donor, proton acceptor, proton donor, electrostatic stabiliser |
| His72 | His42A | Deprotonates hydrogen peroxide to give singly ionised Fe(III)-coordinated peroxide. Protonates the departing hydroxide ion during heterolytic cleavage of the O-O bond in the coordinated peroxide. Accepts a proton from the phenol substrate (via an active site water molecule). Protonates the Fe-coordinated oxygen in the final step of the reaction. | hydrogen bond acceptor, hydrogen bond donor, proton acceptor, proton donor, electrostatic stabiliser |
| His200 | His170A | Acts as the heme axial ligand. | metal ligand |
| Asn100 | Asn70A | Modifies the pKa of His 42. | increase basicity, hydrogen bond acceptor, electrostatic stabiliser, increase acidity |
Chemical Components
proton transfer, bimolecular nucleophilic substitution, coordination to a metal ion, decoordination from a metal ion, overall reactant used, heterolysis, electron transfer, radical formation, redox reaction, cofactor used, rate-determining step, radical propagation, intermediate formation, overall product formed, proton relay, native state of cofactor regenerated, native state of enzyme regeneratedReferences
- Meno K et al. (2002), Acta Crystallogr D Biol Crystallogr, 58, 1803-1812. Structural analysis of the two horseradish peroxidase catalytic residue variants H42E and R38S/H42E: implications for the catalytic cycle. DOI:10.1107/s090744490201329x. PMID:12351824.
- Derat E et al. (2006), J Am Chem Soc, 128, 13940-13949. An Efficient Proton-Coupled Electron-Transfer Process during Oxidation of Ferulic Acid by Horseradish Peroxidase: Coming Full Cycle. DOI:10.1021/ja065058d. PMID:17044722.
- Derat E et al. (2006), J Phys Chem B, 110, 10526-10533. The Poulos−Kraut Mechanism of Compound I Formation in Horseradish Peroxidase: A QM/MM Study. DOI:10.1021/jp055412e. PMID:16722763.
- Gajhede M (2001), Biochem Soc Trans, 29, 91-98. Plant peroxidases: substrate complexes with mechanistic implications. DOI:10.1042/0300-5127:0290091. PMID:11356134.
- Henriksen A et al. (1999), J Biol Chem, 274, 35005-35011. The Structures of the Horseradish Peroxidase C-Ferulic Acid Complex and the Ternary Complex with Cyanide Suggest How Peroxidases Oxidize Small Phenolic Substrates. DOI:10.1074/jbc.274.49.35005. PMID:10574977.

Step 1. His42 deprotonates hydrogen peroxide. The resulting anion coordinates to the Fe(III) centre of Hem350, displacing water and forming Cpd0.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His42A | hydrogen bond acceptor, hydrogen bond donor |
| Arg38A | hydrogen bond donor, electrostatic stabiliser |
| Asn70A | increase basicity, hydrogen bond acceptor, electrostatic stabiliser |
| His170A | metal ligand |
| His42A | proton acceptor |
Chemical Components
proton transfer, ingold: bimolecular nucleophilic substitution, coordination to a metal ion, decoordination from a metal ion, overall reactant used
Step 2. The O-O bond of Cpd0 undergoes heterolytic cleavage and the resulting hydroxide is protonated by His42. The porphryin ring and Fe(III) donate an electron each to the oxygen bound to the metal.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His42A | hydrogen bond donor, electrostatic stabiliser |
| Arg38A | hydrogen bond donor, electrostatic stabiliser |
| Asn70A | hydrogen bond acceptor, increase acidity |
| His170A | metal ligand |
| His42A | proton donor |
Chemical Components
heterolysis, proton transfer, electron transfer, radical formation, redox reaction, cofactor used, rate-determining step
Step 3. His42 deprotonates water, which then deprotonates the phenolic derivative substrate. The phenolate transfers an electron to the porphyrin ring, forming a phenolic radical and Cpd1.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg38A | hydrogen bond donor, electrostatic stabiliser |
| His42A | hydrogen bond acceptor, hydrogen bond donor |
| Asn70A | hydrogen bond acceptor, increase basicity, electrostatic stabiliser |
| His170A | metal ligand |
| His42A | proton acceptor |
Chemical Components
proton transfer, electron transfer, radical propagation, redox reaction, intermediate formation, overall product formed, overall reactant used, proton relay
Step 4. The oxygen of Cpd1 is protonated by water, which is in turn protonated by His42.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His42A | hydrogen bond donor |
| Arg38A | hydrogen bond donor, electrostatic stabiliser |
| Asn70A | hydrogen bond acceptor, increase acidity |
| His170A | metal ligand |
| His42A | proton donor |
Chemical Components
proton transfer, proton relay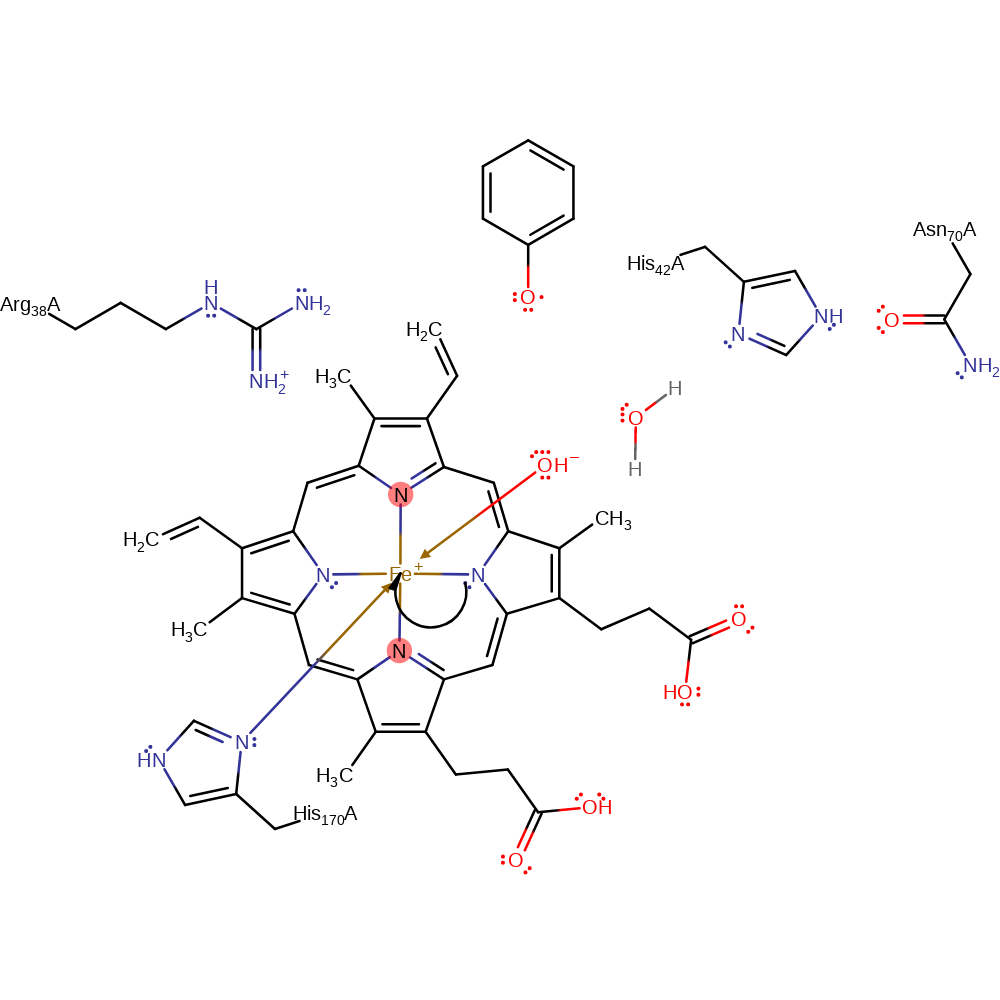
Step 5. The porphyrin ring transfers an electron to the Fe(IV) centre to give an isomer of Cpd1.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His42A | hydrogen bond donor, hydrogen bond acceptor |
| Arg38A | hydrogen bond donor |
| Asn70A | hydrogen bond acceptor |
| His170A | metal ligand |
Chemical Components
electron transfer, radical propagation, intermediate formationCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His42A | hydrogen bond donor, hydrogen bond acceptor |
| Arg38A | hydrogen bond donor |
| Asn70A | hydrogen bond acceptor |
| His170A | metal ligand |
| Arg38A | proton donor |
Chemical Components
proton transfer
Step 7. Upon binding of the second phenol derivative, Arg38 deprotonates Cpd2. The doubly-protonated form of Cpd2 is less stable in the presence of the phenol derivative than in the presence of the phenolic radical. Therefore when the phenolic radical product dissociates and the phenolic derivative substrate associates, there is proton transfer to form the more stable isomer of Cpd2.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His170A | metal ligand |
| His42A | hydrogen bond acceptor, hydrogen bond donor |
| Arg38A | hydrogen bond acceptor |
| Asn70A | hydrogen bond acceptor |
| Arg38A | proton acceptor |
Chemical Components
proton transfer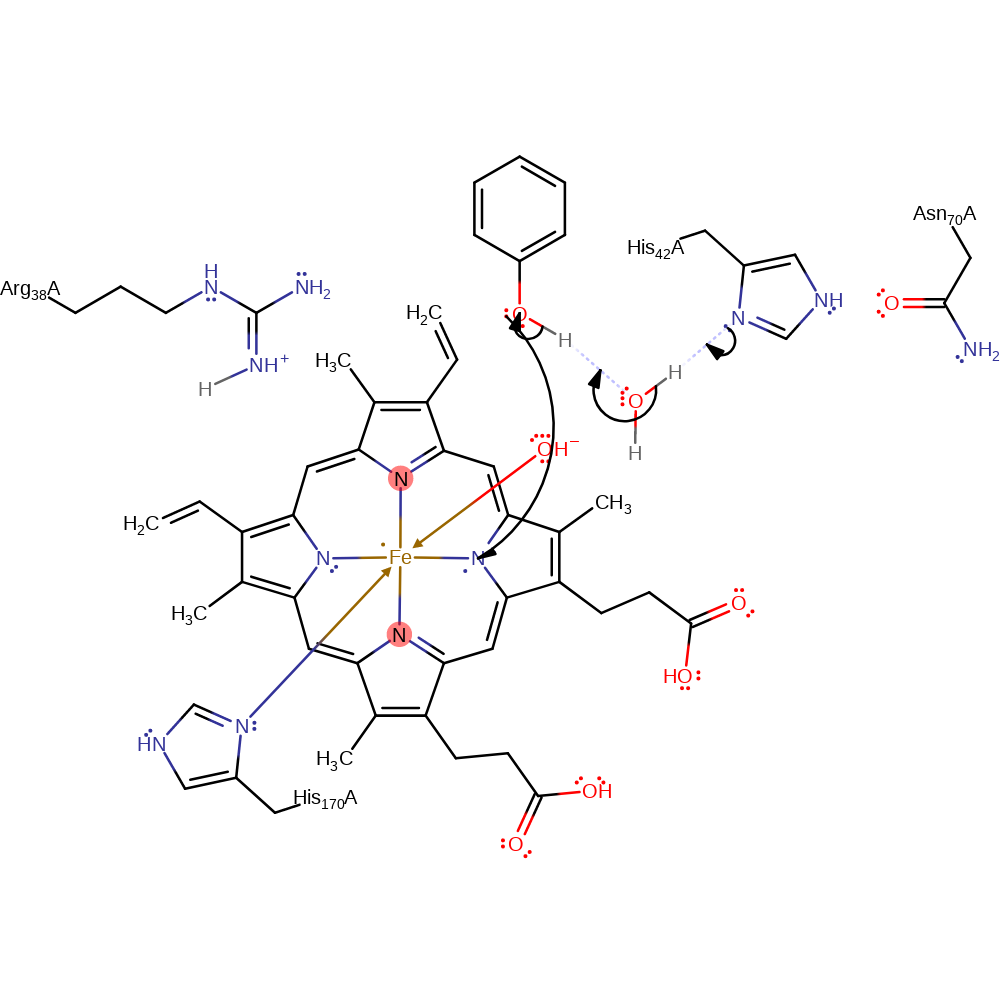
Step 8. His42 deprotonates water, which then deprotonates the phenolic derivative substrate. The phenolate transfers an electron to the porphyrin ring radical, forming a phenolic radical.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His170A | metal ligand |
| His42A | hydrogen bond acceptor, hydrogen bond donor |
| Arg38A | electrostatic stabiliser, hydrogen bond donor |
| Asn70A | hydrogen bond acceptor, electrostatic stabiliser, increase basicity |
| His42A | proton acceptor |
Chemical Components
proton transfer, electron transfer, radical propagation, native state of cofactor regenerated, overall product formed, overall reactant used, proton relay
Step 9. The metal-bound hydroxide is protonated by water, which is in turn protonated by His42.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His42A | hydrogen bond donor |
| Arg38A | electrostatic stabiliser, hydrogen bond donor |
| Asn70A | increase acidity, hydrogen bond acceptor |
| His170A | metal ligand |
| His42A | proton donor |
Chemical Components
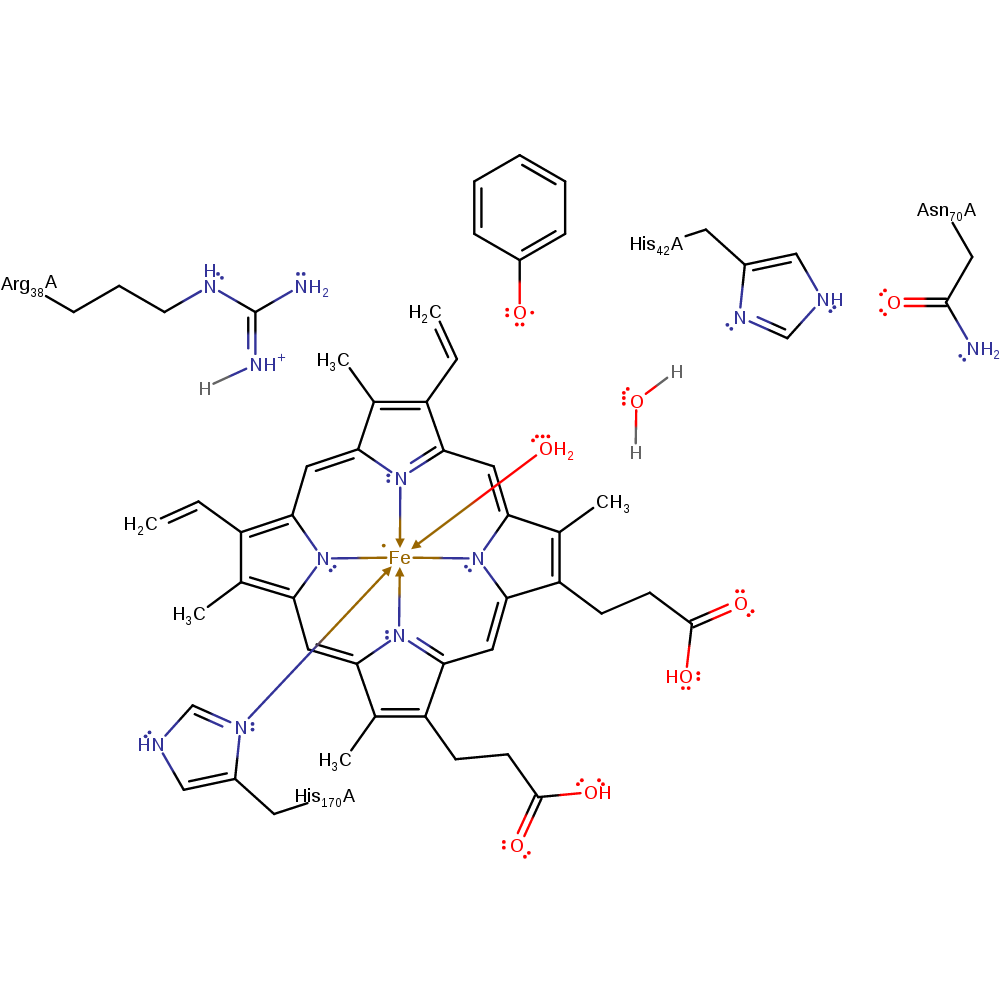
proton transfer, native state of enzyme regenerated, proton relayIntroduction
The reaction occurs in three steps:
The enzyme is first oxidised by hydrogen peroxide and is then reduced in two sequential one-electron transfer steps from reducing substrates (typically small molecule phenol derivatives). The mechanism of the first step is believed to be common to all haem peroxidases. HOOH is deprotonated by a water molecule which is deprotonated by His 42 to produce singly ionised Fe(III)-coordinated peroxide. The O-O bond of the coordinated peroxide is now heterolytically cleaved to generate an Fe(IV)=O species and a hydroxide ion, which is protonated by His 42. Arg 38 is though to promote the reaction by stabilising negative charge in the transient Fe(III)-OOH intermediate and associated transition states.
In the second step, a reducing phenol substrate binds to the active site and transfers an electron to haem and a proton to His 42. The proton transfer is thought to occur via an active site water molecule, and is proposed to be assisted by Arg 38 which forms a hydrogen bond to the substrate phenolic oxygen.
The product radical now dissociates from the active site and a second reducing phenol substrate is bound. This second reducing substrate transfers an electron to the haem, and a proton (via an active site water) to the Fe-coordinated O atom. This is accompanied by proton transfer from His 42 to the Fe-coordinated oxygen, so that the overall result is formation of a water molecule and regeneration of the resting state haem Fe(III).
Catalytic Residues Roles
| UniProt | PDB* (7atj) | ||
| Arg68 | Arg38A | Stabilises negative charge in the transient Fe(III)-OOH intermediate and associated transition states. Assists proton transfer from the phenol substrates to either His 42 or the ferryl oxygen. | hydrogen bond acceptor, hydrogen bond donor, proton acceptor, proton donor, electrostatic stabiliser |
| His72 | His42A | Deprotonates a water molecule which deprotonates hydrogen peroxide to give singly ionised Fe(III)-coordinated peroxide. Protonates the departing hydroxide ion during heterolytic cleavage of the O-O bond in the coordinated peroxide. Accepts a proton from the phenol substrate (via an active site water molecule). Protonates the Fe-coordinated oxygen in the final step of the reaction. | hydrogen bond acceptor, hydrogen bond donor, proton acceptor, proton donor, electrostatic stabiliser |
| His200 | His170A | Acts as the heme axial ligand. | metal ligand |
| Asn100 | Asn70A | Modifies the pKa of His 42. | increase basicity, hydrogen bond acceptor, electrostatic stabiliser, increase acidity |
Chemical Components
proton transfer, coordination to a metal ion, decoordination from a metal ion, overall reactant used, rate-determining step, heterolysis, electron transfer, radical formation, redox reaction, cofactor used, radical propagation, intermediate formation, overall product formed, proton relay, native state of cofactor regenerated, native state of enzyme regeneratedReferences
- Meno K et al. (2002), Acta Crystallogr D Biol Crystallogr, 58, 1803-1812. Structural analysis of the two horseradish peroxidase catalytic residue variants H42E and R38S/H42E: implications for the catalytic cycle. DOI:10.1107/s090744490201329x. PMID:12351824.
- Vidossich P et al. (2010), J Phys Chem B, 114, 5161-5169. On the role of water in peroxidase catalysis: a theoretical investigation of HRP compound I formation. DOI:10.1021/jp911170b. PMID:20345187.
- Gajhede M (2001), Biochem Soc Trans, 29, 91-98. Plant peroxidases: substrate complexes with mechanistic implications. DOI:10.1042/0300-5127:0290091. PMID:11356134.
- Henriksen A et al. (1999), J Biol Chem, 274, 35005-35011. The Structures of the Horseradish Peroxidase C-Ferulic Acid Complex and the Ternary Complex with Cyanide Suggest How Peroxidases Oxidize Small Phenolic Substrates. DOI:10.1074/jbc.274.49.35005. PMID:10574977.
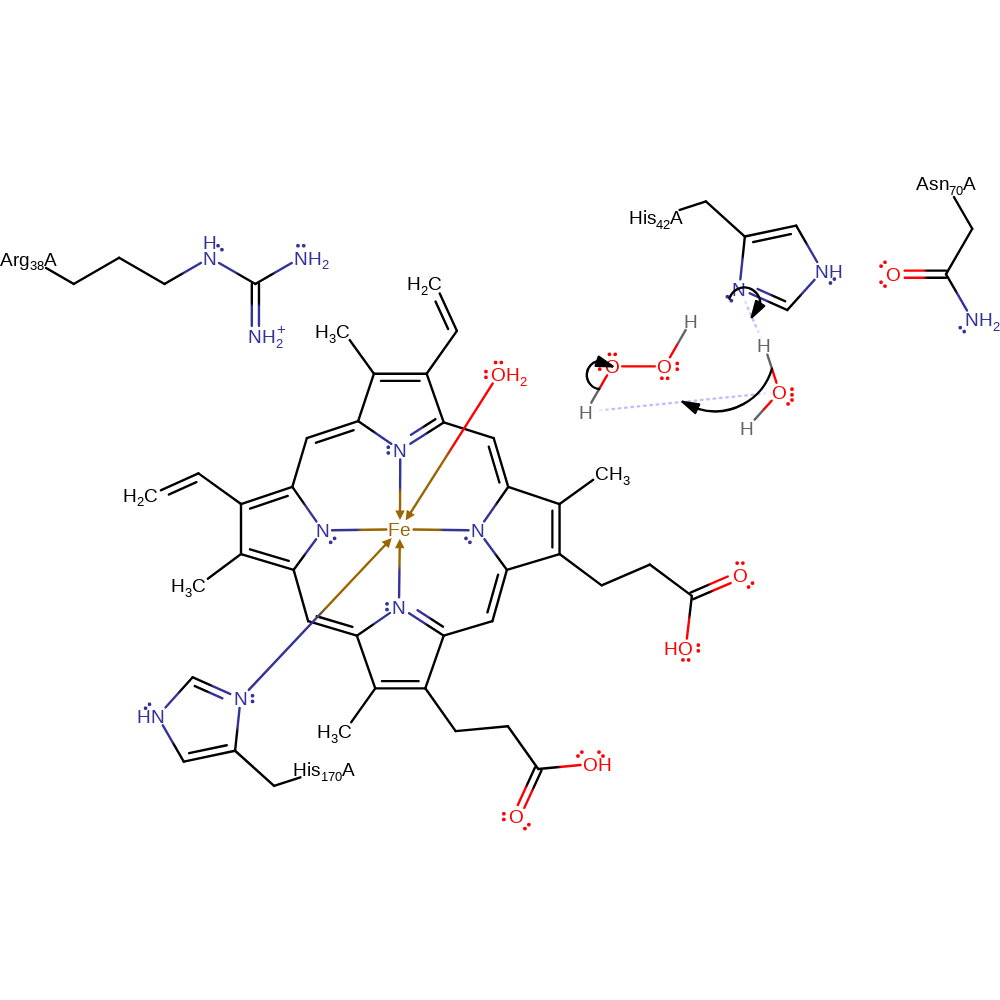
Step 1. His42 deprotonates a water molecule which deprotonates hydrogen peroxide. The resulting anion coordinates to the Fe(III) centre of Hem350, displacing water and forming Cpd0.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His170A | metal ligand |
| His42A | hydrogen bond donor, hydrogen bond acceptor |
| Asn70A | hydrogen bond acceptor |
| Arg38A | hydrogen bond donor, electrostatic stabiliser |
| Asn70A | increase basicity, electrostatic stabiliser |
| His42A | proton acceptor |
Chemical Components
proton transfer, coordination to a metal ion, decoordination from a metal ion, overall reactant used
Step 2. The O-O bond of Cpd0 undergoes heterolytic cleavage and the resulting hydroxide is protonated by His42. The porphryin ring and Fe(III) donate an electron each to the oxygen bound to the metal.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg38A | electrostatic stabiliser |
| His42A | electrostatic stabiliser |
| Arg38A | hydrogen bond donor |
| His42A | hydrogen bond donor |
| Asn70A | hydrogen bond acceptor |
| His170A | metal ligand |
| Asn70A | increase acidity |
| His42A | proton donor |
Chemical Components
rate-determining step, heterolysis, proton transfer, electron transfer, radical formation, redox reaction, cofactor used
Step 3. His42 deprotonates water, which then deprotonates the phenolic derivative substrate. The phenolate transfers an electron to the porphyrin ring, forming a phenolic radical and Cpd1.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His170A | metal ligand |
| His42A | hydrogen bond acceptor |
| Arg38A | hydrogen bond donor |
| Asn70A | hydrogen bond acceptor, increase basicity, electrostatic stabiliser |
| His42A | proton acceptor |
Chemical Components
proton transfer, electron transfer, radical propagation, redox reaction, intermediate formation, overall product formed, overall reactant used, proton relay
Step 4. The oxygen of Cpd1 is protonated by water, which is in turn protonated by His42.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His170A | metal ligand |
| His42A | hydrogen bond donor |
| Arg38A | hydrogen bond donor |
| Asn70A | hydrogen bond acceptor, increase acidity |
| His42A | proton donor |
Chemical Components
proton transfer, proton relay
Step 5. The porphyrin ring transfers an electron to the Fe(IV) centre to give an isomer of Cpd1.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His170A | metal ligand |
| Arg38A | hydrogen bond donor |
| His42A | hydrogen bond donor, hydrogen bond acceptor |
| Asn70A | hydrogen bond acceptor |
Chemical Components
electron transfer, radical propagation, intermediate formationCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His170A | metal ligand |
| Arg38A | hydrogen bond donor |
| His42A | hydrogen bond donor |
| Asn70A | hydrogen bond acceptor |
| Arg38A | proton donor |
Chemical Components
proton transfer
Step 7. Upon binding of the second phenol derivative, Arg38 deprotonates Cpd2. The doubly-protonated form of Cpd2 is less stable in the presence of the phenol derivative than in the presence of the phenolic radical. Therefore when the phenolic radical product dissociates and the phenolic derivative substrate associates, there is proton transfer to form the more stable isomer of Cpd2.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His170A | metal ligand |
| Arg38A | hydrogen bond acceptor |
| His42A | hydrogen bond acceptor |
| Asn70A | hydrogen bond acceptor |
| His42A | hydrogen bond donor |
| Arg38A | proton acceptor |
Chemical Components
proton transfer
Step 8. His42 deprotonates water, which then deprotonates the phenolic derivative substrate. The phenolate transfers an electron to the porphyrin ring radical, forming a phenolic radical.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His170A | metal ligand |
| Arg38A | hydrogen bond donor |
| His42A | hydrogen bond donor, hydrogen bond acceptor |
| Asn70A | hydrogen bond acceptor |
| Asn70A | increase acidity, electrostatic stabiliser |
| His42A | proton acceptor |
Chemical Components
proton transfer, electron transfer, radical propagation, native state of cofactor regenerated, overall product formed, overall reactant used, proton relay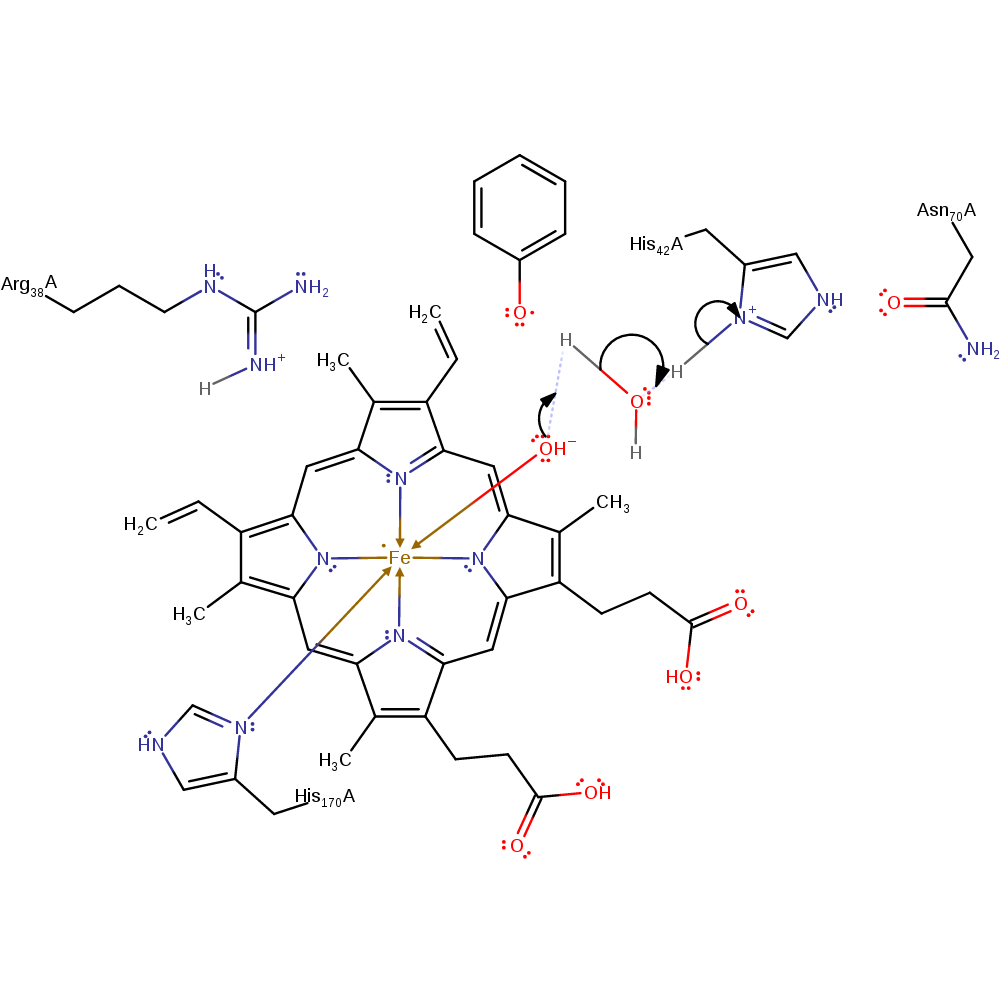
Step 9. The metal-bound hydroxide is protonated by water, which is in turn protonated by His42.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His170A | metal ligand |
| Arg38A | hydrogen bond donor |
| His42A | hydrogen bond donor |
| Asn70A | hydrogen bond acceptor, increase acidity |
| His42A | proton donor |





 Download:
Download: 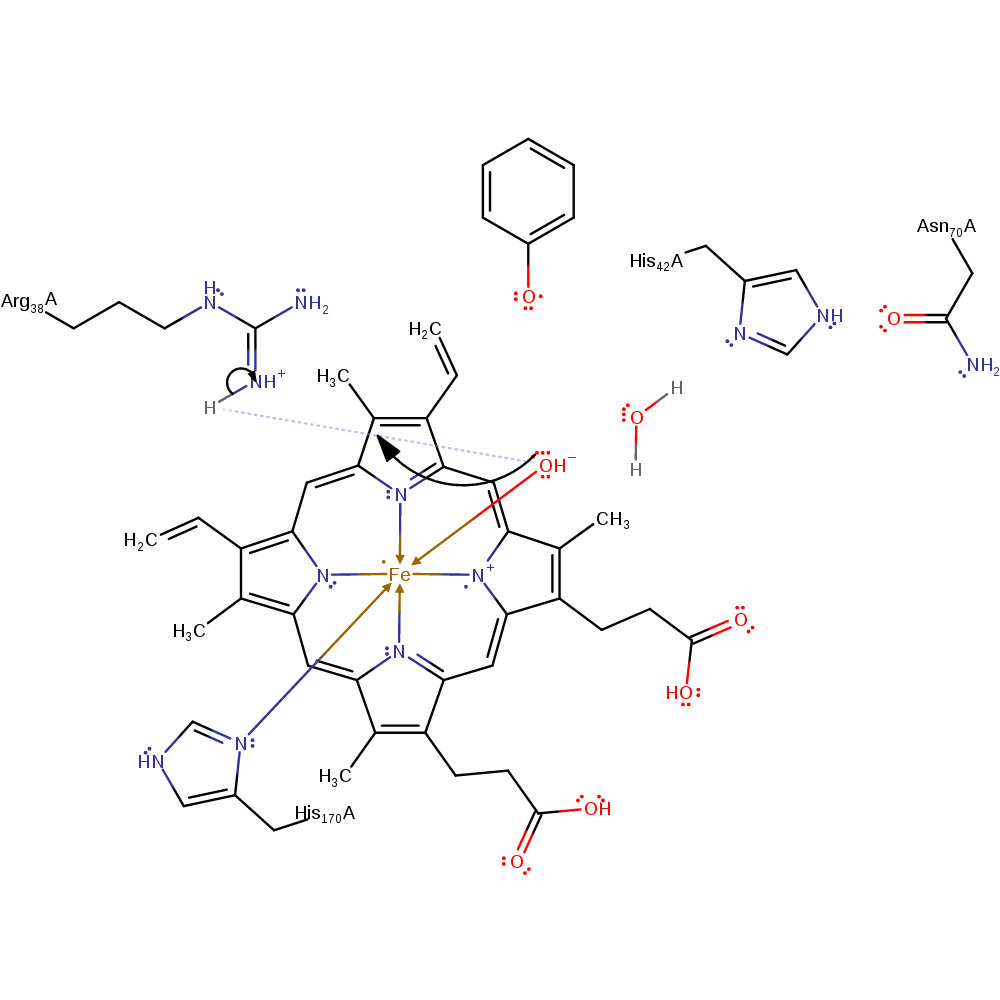
 Download:
Download: