Transketolase
Transketolase (EC:2.2.1.1) (TK) catalyses the reversible transfer of a two-carbon ketol unit from xylulose 5-phosphate to an aldose receptor, such as ribose 5-phosphate, to form sedoheptulose 7-phosphate and glyceraldehyde 3- phosphate. This enzyme, together with transaldolase, provides a link between the glycolytic and pentose-phosphate pathways. TK requires thiamine pyrophosphate and a divalent metal cation as a cofactor.
Catalytic activity has been noted with a wide variety of divalent metal ions coordinating the diphosphate group of the cofactor [PMID:9924800] and whilst there is still some debate as to the exact nature of the native metal cation (most of the work on the determination of the mechanism has utilised the magnesium cation), Esakova et al. have determined that the enzyme is more stable in the presence of Ca(II), making this the more likely native metal cation [PMID:16125202] .
Reference Protein and Structure
- Sequence
-
P29401
 (2.2.1.1)
(2.2.1.1)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Homo sapiens (Human)

- PDB
-
4kxv
- Human transketolase in covalent complex with donor ketose D-xylulose-5-phosphate, crystal 1
(0.97 Å)



- Catalytic CATH Domains
-
3.40.50.970
 (see all for 4kxv)
(see all for 4kxv)
- Cofactors
- Magnesium(2+) (1), Thiamine(1+) diphosphate(3-) (1) Metal MACiE
Enzyme Reaction (EC:2.2.1.1)
Enzyme Mechanism
Introduction
Glu418B activates the thiamine diphosphate cofactor by abstracting a proton from the NH group of the 6-membered ring. This results in double bond rearrangement and the abstraction of a proton from the N=CH-S moiety. The carbanion of thiamine diphosphate then attacks the carbonyl carbon of the sugar (in its open form) in a nucleophilic addition that results in the cofactor undergoing another double bond rearrangement and abstracting the proton back from Glu418B. His263A deprotonates the 2-OH group of the covaltnely attached intermediate, initiating the elimination of D-xylulose 5-phosphate. Thaimine diphosphate acts as an electron sink. Thiamine diphosphate initiates a double bond rearrangement, which results in the intermediate attacking (2R)-2-Hydroxy-3-(phosphonooxy)-propanal in a nucleophilic addition. The formed oxyanion deprotonates His263A. Glu418B deprotonates thiamine diphosphate, which initiates a double bond rearrangement, that deprotonates the hydroxide of the intermediate, and results in a reformation of the carbanionic activated cofactor and the D-xylulose 5-phosphate product. The carbanion of the thiamine diphosphate cofactor deprotonates the adjacent amine, which initiates double bond rearrangement that results in the deprotonation of Glu418B. Cyclisation of the sugar product occurs spontaneously outside of the enzyme's active site.
Catalytic Residues Roles
| UniProt | PDB* (4kxv) | ||
| Glu418 | Glu418B | Acts as a general acid/base, important for activating the thiamine diphosphate cofactor. | hydrogen bond acceptor, hydrogen bond donor, proton acceptor, proton donor |
| His263 | His263A | Acts as a general acid/base. | hydrogen bond acceptor, hydrogen bond donor, proton acceptor, proton donor |
| His30, His481 | His30A, His481B | Help bind and stabilise the substrate and reactive intermediates formed during the course of the reaction. | activator, hydrogen bond donor, steric role |
Chemical Components
proton transfer, assisted tautomerisation (not keto-enol), cofactor used, bimolecular nucleophilic addition, aldol addition, overall reactant used, intermediate formation, bimolecular elimination, overall product formed, intermediate collapse, intermediate terminated, native state of cofactor regenerated, native state of enzyme regenerated, reaction occurs outside the enzyme, cyclisation, intramolecular nucleophilic additionReferences
- Wikner C et al. (1997), Biochemistry, 36, 15643-15649. Identification of Catalytically Important Residues in Yeast Transketolase†. DOI:10.1021/bi971606b. PMID:9398292.
- Nauton L et al. (2016), Biochemistry, 55, 2144-2152. Insights into the Thiamine Diphosphate Enzyme Activation Mechanism: Computational Model for Transketolase Using a Quantum Mechanical/Molecular Mechanical Method. DOI:10.1021/acs.biochem.5b00787. PMID:26998737.
- Tittmann K (2014), Bioorg Chem, 57, 263-280. Sweet siblings with different faces: the mechanisms of FBP and F6P aldolase, transaldolase, transketolase and phosphoketolase revisited in light of recent structural data. DOI:10.1016/j.bioorg.2014.09.001. PMID:25267444.
- Sheng X et al. (2013), J Mol Graph Model, 39, 23-28. Theoretical studies on the common catalytic mechanism of transketolase by using simplified models. DOI:10.1016/j.jmgm.2012.11.001. PMID:23220278.
- Esakova OA et al. (2005), Life Sci, 78, 8-13. Effects of transketolase cofactors on its conformation and stability. DOI:10.1016/j.lfs.2004.12.055. PMID:16125202.
- Schenk G et al. (1998), Int J Biochem Cell Biol, 30, 1297-1318. Properties and functions of the thiamin diphosphate dependent enzyme transketolase. DOI:10.1016/s1357-2725(98)00095-8. PMID:9924800.

Step 1. Glu418B deprotonates the thiamine diphosphate cofactor, which initiates double bond rearrangement that results in the deprotonation of the N=CH-S group, activating the cofactor. The double bonds can rearrage either as described here, or anti-clockwise around the aromatic ring.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His481B | electrostatic stabiliser, hydrogen bond acceptor |
| His263A | hydrogen bond donor |
| Glu418B | hydrogen bond acceptor |
| Glu418B | proton acceptor |
Chemical Components
proton transfer, assisted tautomerisation (not keto-enol), cofactor used
Step 2. The carbanion of thiamine diphosphate attacks the carbonyl carbon of sedoheptulose 7-phosphate in a nucleophilic addition that results in the cofactor undergoing double bond rearrangement that results in the deprotonation of Glu418B.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu418B | hydrogen bond donor |
| His481B | electrostatic stabiliser, hydrogen bond acceptor |
| His30A | hydrogen bond donor |
| His263A | hydrogen bond donor |
| Glu418B | proton donor |
Chemical Components
ingold: bimolecular nucleophilic addition, proton transfer, aldol addition, overall reactant used, intermediate formation
Step 3. His263A deprotonates the 2-OH group of the covalently attached intermediate, initiating the elimination of D-xylulose 5-phosphate. Thaimine diphosphate acts as an electron sink. His30 facilitates the proton transfer to His263 by a hydrogen bond to the hydroxyl group, positioning it in the correct orientation and stabilising the developing negative charge of the transition state [PMID:9398292]. The cofactor acts as an electron sink.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu418B | hydrogen bond acceptor |
| His481B | electrostatic stabiliser, hydrogen bond acceptor |
| His30A | hydrogen bond donor, activator, steric role |
| His263A | hydrogen bond donor, hydrogen bond acceptor |
| His263A | proton acceptor |
Chemical Components
ingold: bimolecular elimination, assisted tautomerisation (not keto-enol), overall product formed, intermediate formation, intermediate collapse
Step 4. Thiamine diphosphate initiates a double bond rearrangement, which results in the intermediate attacking (2R)-2-Hydroxy-3-(phosphonooxy)-propanal in a nucleophilic addition. The formed oxyanion deprotonates His263A.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu418B | hydrogen bond acceptor |
| His481B | electrostatic stabiliser, hydrogen bond acceptor |
| His30A | hydrogen bond donor, activator, steric role |
| His263A | hydrogen bond donor |
| His263A | proton donor |
Chemical Components
ingold: bimolecular nucleophilic addition, assisted tautomerisation (not keto-enol), aldol addition, overall reactant used, intermediate formation
Step 5. Glu418B deprotonates thiamine diphosphate, which initiates a double bond rearrangement, that deprotonates the hydroxide of the intermediate, and results in a reformation of the carbanionic activated cofactor and the D-xylulose-5-phosphate product.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu418B | hydrogen bond acceptor |
| His481B | electrostatic stabiliser, hydrogen bond acceptor |
| His30A | hydrogen bond donor |
| His263A | hydrogen bond donor |
| Glu418B | proton acceptor |
Chemical Components
proton transfer, ingold: bimolecular elimination, assisted tautomerisation (not keto-enol), overall product formed, intermediate collapse, intermediate formation
Step 6. The carbanion of the thiamine diphosphate cofactor deprotonates the adjacent amine, which initiates double bond rearrangement that results in the deprotonation of Glu418B.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu418B | hydrogen bond donor |
| His481B | electrostatic stabiliser, hydrogen bond acceptor |
| His30A | hydrogen bond donor |
| His263A | hydrogen bond donor |
| Glu418B | proton donor |
Chemical Components
proton transfer, assisted tautomerisation (not keto-enol), intermediate terminated, native state of cofactor regenerated, native state of enzyme regenerated
Step 7. The product of the enzyme is the linear molecule. The cyclisation is not enzyme catalysed, and has been inferred from the overall reaction products.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|
Chemical Components
reaction occurs outside the enzyme, cyclisation, ingold: intramolecular nucleophilic addition, proton transferIntroduction
Activated carbanion of thiamine diphosphate (ThDP) cofactor performs a nucleophilic attack towards the D-xylulose-5-phosphate, resulting in double bond rearrangement concerted with deprotonation of the ThDP's amine group by the substrate. Lys244 deprotonates His258 which then deprotonates the 2-OH group of the covalently attached intermediate, initiating the elimination of D-glyceraldehyde 3-phosphate. Thiamine diphosphate acts as an electron sink. Thiamine diphosphate initiates a double bond rearrangement, which results in the intermediate attacking the aldose D-erythrose-4-phosphate substrate in a nucleophilic addition. This results in a more negative carbonyl oxygen which is subsequently implicated in a series of proton transfers for restoration of the initial protonation state of His258 and Lys244 catalytic residues. ThDP cofactor initiates a double bond rearrangement that deprotonates the hydroxide of the intermediate, and results in a reformation of the carbanionic activated cofactor and the D-fructose-6-phosphate product.
Catalytic Residues Roles
| UniProt | PDB* (4kxv) | ||
| His258 | His258A | His258 operates as the general base and acid to mediate proton transfer by accepting a proton from the O3H group of the substrate and delivering another one to Lys244. | proton acceptor, proton donor |
| Lys244 | Lys244A | Involve in acid-base catalysis by accepting and donating protons from His258. | proton acceptor, proton donor |
Chemical Components
bimolecular nucleophilic addition, proton transfer, bimolecular elimination, assisted keto-enol tautomerisation, intermediate collapse, intermediate formation, overall product formed, overall reactant used, assisted tautomerisation (not keto-enol), aldol additionReferences
- Prejanò M et al. (2019), Chemphyschem, 20, 2881-2886. The Catalytic Mechanism of Human Transketolase. DOI:10.1002/cphc.201900650. PMID:31489766.
- Prejanò M et al. (2020), ACS Catal, 10, 2872-2881. How the Destabilization of a Reaction Intermediate Affects Enzymatic Efficiency: The Case of Human Transketolase. DOI:10.1021/acscatal.9b04690. PMID:33828899.

Step 1. The carbanion of thiamine diphosphate (ThDP) attacks the carbonyl carbon of D-xylulose-5-phosphate in a nucleophilic addition that results in the cofactor undergoing double bond rearrangement. Simultaneously, the carbonyl oxygen of D-xylulose-5-phosphate deprotonates the amine group of the ThDP cofactor.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|
Chemical Components
ingold: bimolecular nucleophilic addition, proton transfer
Step 2. Lys244 deprotonates His258, thereby activating it. Activated His258 deprotonates the 2-OH group of the covalently attached intermediate, initiating the elimination of D-glyceraldehyde 3-phosphate via cleavage of the C-C scissile bond. Thiamine diphosphate acts as an electron sink.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Lys244A | proton acceptor |
| His258A | proton donor, proton acceptor |
Chemical Components
ingold: bimolecular elimination, assisted keto-enol tautomerisation, intermediate collapse, intermediate formation, overall product formedCatalytic Residues Roles
| Residue | Roles |
|---|
Chemical Components
overall product formed
Step 4. Thiamine diphosphate initiates a double bond rearrangement, which results in the intermediate attacking the aldose D-erythrose-4-phosphate substrate in a nucleophilic addition. This resulted in a more negative carbonyl oxygen which is subsequently implicated in a series of proton transfers for restoration of the initial protonation state of His258 and Lys244 catalytic residues.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Lys244A | proton donor |
| His258A | proton donor, proton acceptor |
Chemical Components
ingold: bimolecular nucleophilic addition, overall reactant used, intermediate formation, assisted tautomerisation (not keto-enol), aldol addition
Step 5. ThDP cofactor initiates a double bond rearrangement, that deprotonates the hydroxide of the intermediate, and results in a reformation of the carbanionic activated cofactor and the D-fructose-6-phosphate product.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|




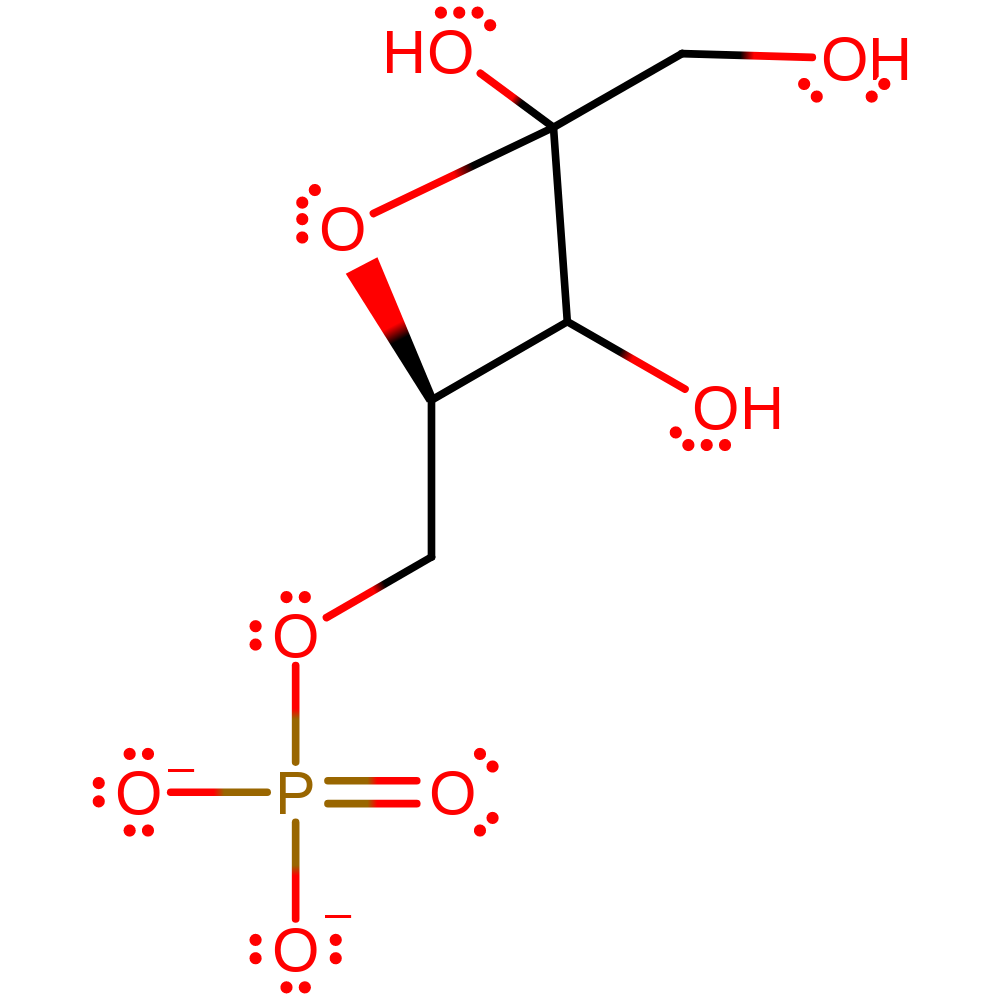 Download:
Download: 
 Download:
Download: