Taurine dioxygenase
Taurine:alpha-ketoglutarate dioxygenase (TauD) hydroxylates C1 of taurine (2-aminoethane-1-sulfonic acid) and other organosulfates, leading to elimination of sulfite and thereby initiating the acquisition of sulphur from compounds that contain the element in a form that would otherwise be biologically inert. Escherichia coli expresses TauD only in the absence of sulfate. The reaction requires molecular dioxygen and 2-oxoglutarate as a cosubstrate.
Reference Protein and Structure
- Sequence
-
P37610
 (1.14.11.17)
(1.14.11.17)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Escherichia coli K-12 (Bacteria)

- PDB
-
1os7
- Crystal structure of TauD with iron, alpha-ketoglutarate and Taurine bound at pH 7.5
(2.5 Å)



- Catalytic CATH Domains
-
3.60.130.10
 (see all for 1os7)
(see all for 1os7)
- Cofactors
- Iron(2+) (1) Metal MACiE
Enzyme Reaction (EC:1.14.11.17)
Enzyme Mechanism
Introduction
In the resting state, Fe(II) is coordinated by His99, Asp 101, His 255 and three water molecules. The cosubstrate 2-oxoglutarate binds to Fe(II) via the carbonyls on C1 and C2, displacing two water molecules. Taurine binds (not to Fe(II)), displacing the third water molecule. Dioxygen binds to Fe(II) via one of the oxygen atoms; this formally oxidises iron to Fe(III). The radical on the O-O bond attacks C2 of 2-oxoglutarate, with the C2 carbonyl attacking and oxidising iron to Fe(IV). The cosubstrate is decarboxylated with C1 leaving as carbon dioxide. This is concomitant with heterolytic O-O bond cleavage and oxidation of the cosubstrate, giving an Fe(IV)=O oxoferryl intermediate with bound succinate. The oxo atom abstracts the taurine C1 hydrogen in a radical mechanism, with reduction of iron to Fe(III)-OH, leaving a radical on taurine. The taurine radical is hydroxylated with OH to give 1-hydroxytaurine, with reduction of iron to Fe(II), regenerating the initial oxidation state. 1-hydroxytaurine decomposes to give sulfite and aminoacetaldehyde. Arg 270 changes its hydrogen bonding to the cosubstrate during the reaction, and may initiate the decarboxylation step or help to remove carbon dioxide from the metal coordination sphere.
Catalytic Residues Roles
| UniProt | PDB* (1os7) | ||
| Arg270 | Arg270B | Arg 270 hydrogen bonds to the co-substrate, and may initiate decarboxylation, or the leaving of carbon dioxide from the iron coordination sphere. | hydrogen bond donor, electrostatic stabiliser |
| His99, Asp101, His255 | His99B, Asp101B, His255B | Forms part of the catalytic iron binding site. | metal ligand |
Chemical Components
bimolecular homolytic addition, redox reaction, radical formation, overall reactant used, cofactor used, coordination to a metal ion, intermediate formation, bimolecular nucleophilic addition, electron transfer, radical termination, unimolecular elimination by the conjugate base, intermediate collapse, overall product formed, decarboxylation, hydrogen transfer, bimolecular homolytic substitution, decoordination from a metal ion, intermediate terminated, native state of cofactor regenerated, native state of enzyme regenerated, reaction occurs outside the enzymeReferences
- Müller I et al. (2004), Biochemistry, 43, 3075-3088. Crystal Structure of the Alkylsulfatase AtsK: Insights into the Catalytic Mechanism of the Fe(II) α-Ketoglutarate-Dependent Dioxygenase Superfamily†,‡. DOI:10.1021/bi035752v. PMID:15023059.
- Martinez S et al. (2015), J Biol Chem, 290, 20702-20711. Catalytic Mechanisms of Fe(II)- and 2-Oxoglutarate-dependent Oxygenases. DOI:10.1074/jbc.R115.648691. PMID:26152721.
- Casey TM et al. (2013), J Phys Chem B, 117, 10384-10394. Measuring the orientation of taurine in the active site of the non-heme Fe(II)/α-ketoglutarate-dependent taurine hydroxylase (TauD) using electron spin echo envelope modulation (ESEEM) spectroscopy. DOI:10.1021/jp404743d. PMID:23937570.
- Knauer SH et al. (2012), FEBS J, 279, 816-831. The Fe(II)/α-ketoglutarate-dependent taurine dioxygenases from Pseudomonas putida and Escherichia coli are tetramers. DOI:10.1111/j.1742-4658.2012.08473.x. PMID:22221834.
- Grzyska PK et al. (2010), Proc Natl Acad Sci U S A, 107, 3982-3987. Insight into the mechanism of an iron dioxygenase by resolution of steps following the FeIV=HO species. DOI:10.1073/pnas.0911565107. PMID:20147623.
- McCusker KP et al. (2009), Proc Natl Acad Sci U S A, 106, 19791-19795. Modular behavior of tauD provides insight into the origin of specificity in alpha-ketoglutarate-dependent nonheme iron oxygenases. DOI:10.1073/pnas.0910660106. PMID:19892731.
- Neidig ML et al. (2007), J Am Chem Soc, 129, 14224-14231. CD and MCD of CytC3 and taurine dioxygenase: role of the facial triad in alpha-KG-dependent oxygenases. DOI:10.1021/ja074557r. PMID:17967013.
- Koehntop KD et al. (2006), J Biol Inorg Chem, 11, 63-72. Self-hydroxylation of taurine/alpha-ketoglutarate dioxygenase: evidence for more than one oxygen activation mechanism. DOI:10.1007/s00775-005-0059-4. PMID:16320009.
- Price JC et al. (2005), Biochemistry, 44, 8138-8147. Kinetic Dissection of the Catalytic Mechanism of Taurine:α-Ketoglutarate Dioxygenase (TauD) fromEscherichia coli†. DOI:10.1021/bi050227c. PMID:15924433.
- Kalliri E et al. (2005), Biochem Biophys Res Commun, 338, 191-197. Kinetic and spectroscopic investigation of CoII, NiII, and N-oxalylglycine inhibition of the FeII/alpha-ketoglutarate dioxygenase, TauD. DOI:10.1016/j.bbrc.2005.08.223. PMID:16165092.
- O'Brien JR et al. (2003), Biochemistry, 42, 5547-5554. Substrate-induced conformational changes in Escherichia coli taurine/alpha-ketoglutarate dioxygenase and insight into the oligomeric structure. DOI:10.1021/bi0341096. PMID:12741810.
- Price JC et al. (2003), Biochemistry, 42, 7497-7508. The first direct characterization of a high-valent iron intermediate in the reaction of an alpha-ketoglutarate-dependent dioxygenase: a high-spin FeIV complex in taurine/alpha-ketoglutarate dioxygenase (TauD) from Escherichia coli. DOI:10.1021/bi030011f. PMID:12809506.
- Elkins JM et al. (2002), Biochemistry, 41, 5185-5192. X-ray crystal structure of Escherichia coli taurine/alpha-ketoglutarate dioxygenase complexed to ferrous iron and substrates. PMID:11955067.
- Eichhorn E et al. (1997), J Biol Chem, 272, 23031-23036. Characterization of -Ketoglutarate-dependent Taurine Dioxygenase from Escherichia coli. DOI:10.1074/jbc.272.37.23031. PMID:9287300.
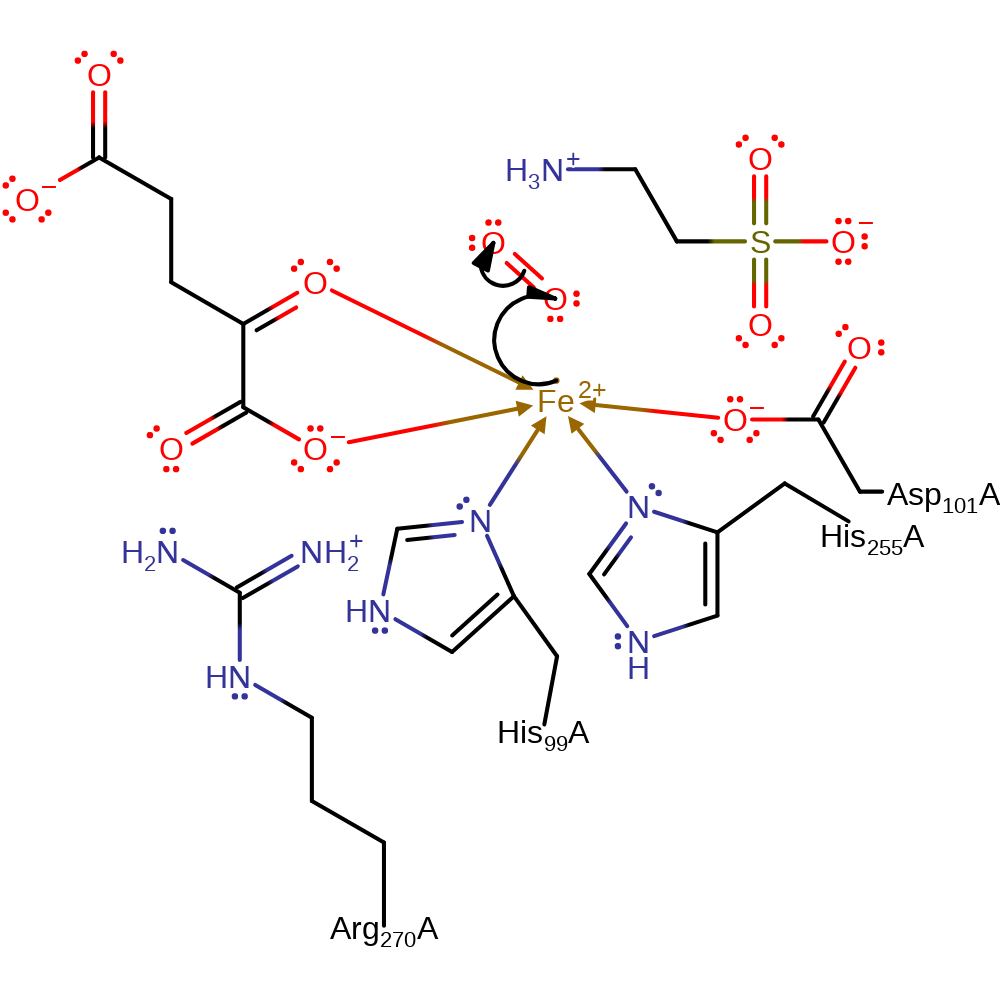
Step 1. Fe(II) donates a single electron to the dioxygen molecule, resulting in the dioxygen being bound to the Fe(III) centre.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg270B | electrostatic stabiliser, hydrogen bond donor |
| His255B | metal ligand |
| His99B | metal ligand |
| Asp101B | metal ligand |
Chemical Components
ingold: bimolecular homolytic addition, redox reaction, radical formation, overall reactant used, cofactor used, coordination to a metal ion, intermediate formation
Step 2. Fe(III) donates a single electron to the bound peroxo group. The terminal oxygen of the peroxo group attacks the carbonyl carbon of the 2-oxoglutarate substrate in a nucleophilic addition.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg270B | electrostatic stabiliser, hydrogen bond donor |
| His255B | metal ligand |
| His99B | metal ligand |
| Asp101B | metal ligand |
Chemical Components
ingold: bimolecular nucleophilic addition, electron transfer, radical termination, overall reactant used, intermediate formation
Step 3. Carbon dioxide is eliminated from the intermediate to form succinate and a iron-bound oxo anion.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg270B | electrostatic stabiliser, hydrogen bond donor |
| His255B | metal ligand |
| His99B | metal ligand |
| Asp101B | metal ligand |
Chemical Components
ingold: unimolecular elimination by the conjugate base, intermediate collapse, intermediate formation, overall product formed, decarboxylation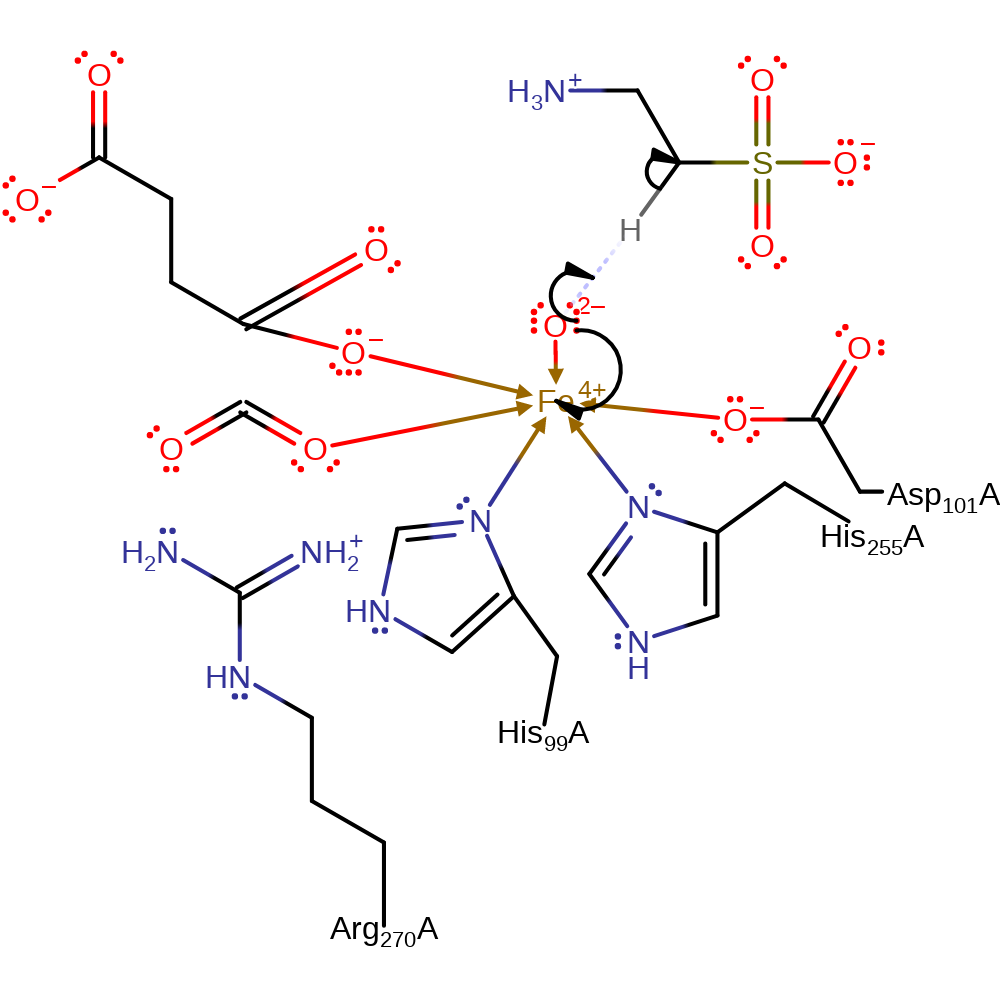
Step 4. The iron-bound oxo anion donates an electron to the Fe(IV) centre and abstracts a proton from the taurine substrate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg270B | hydrogen bond donor |
| His255B | metal ligand |
| His99B | metal ligand |
| Asp101B | metal ligand |
Chemical Components
redox reaction, hydrogen transfer, radical formation, overall reactant used, intermediate formation
Step 5. The carbon radical initiates a homolytic subsitution which forms the 1-hydroxy-2-aminoethanesulfonic acid product and results in a single electron transfer to the Fe(III) centre.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg270B | hydrogen bond donor |
| His255B | metal ligand |
| His99B | metal ligand |
| Asp101B | metal ligand |
Chemical Components
ingold: bimolecular homolytic substitution, radical termination, decoordination from a metal ion, intermediate terminated, overall product formed, native state of cofactor regenerated, native state of enzyme regenerated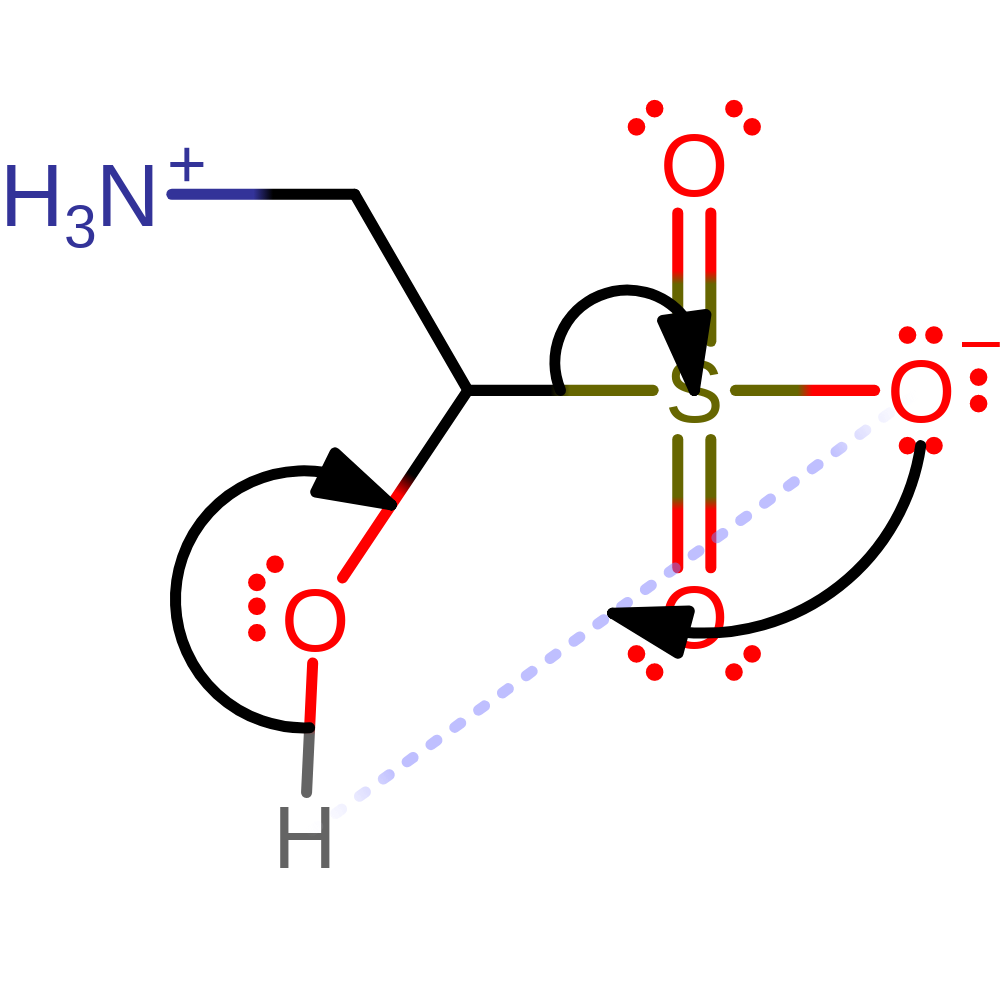
Step 6. The 1-hydroxy-2-aminoethanesulfonic acid decomposes to aminoacetaldehyde and sulfite.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|







 Download:
Download: