Documentation
Step Components
Step components can be described as basic processes involved in the reaction, or an overall
effect which occurs during the course of the reaction. The following are allowable step
components in MACiE:
| Aldol Addition |
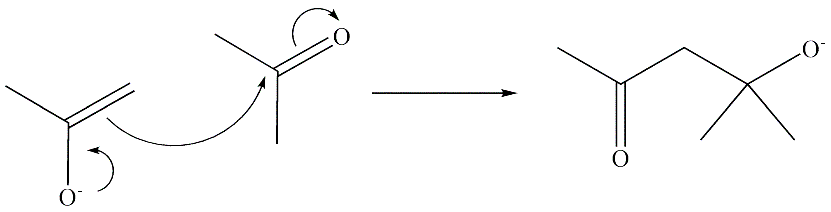
|
| A special case of nucleophilic addition. It is the reaction when the enolate of an aldehyde or a ketone reacts at the alpha-carbon with the carbonyl of another molecule under basic or acidic conditions to obtain a beta-hydroxy aldehyde or ketone. It may be either intramolecular or bimolecular. The figure below shows an example of a bimolecular aldol addition. |
|
| Assisted Keto-Enol Tautomerisation |

|
| Keto-enol tautomerisation assisted by proton transfers. This can either be fully assisted (with two associated proton transfers) or partially assisted (with only one associated proton transfer). |
|
| Assisted Tautomerisation (Not Keto-Enol) |

|
| Tautomerisation other than keto-enol which is assisted by one or more proton transfers. |
|
| Atom Stereo Change |

|
| An atom in the reaction undergoes a change in its sterochemistry. |
|
| Bond Polarisation |

|
| A mechanism component which states that during the course of the associated reaction a charge is formed over a bond, or set of atoms which may involve more than one bond. |
|
| Charge Delocalisation |

|
| A mechanism component which states that during the course of the associated reaction a charge spreads out over a number of bonds in a chemical species. |
|
| Claisen Condensation |

|
| A carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a beta-keto ester or a beta-diketone. |
|
| Claisen Rearrangement |

|
| Highly stereoselective thermally allowed [3,3]-sigmatropic rearrangement of allyl vinyl or allyl aryl ethers. Usually intramolecular, the mechanism is somewhat similar to that of the Diels-Alder. |
|
| Cofactor Used |

|
| A mechanism component which states that during the course of the associated reaction a molecule listed as a cofactor (in the overall reaction) is initially used. |
|
| Colligation |
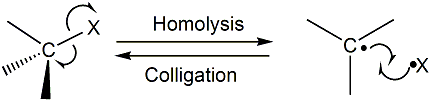
|
| This is the formation of a bond such that each of the molecular fragments between which the bond is formed donate one of the bonding electrons. This is the reverse of a homolysis reaction. |
|
| Coordination |

|
| A reaction in which a single bond is formed by the reaction of two oppositely charged ions. This is the reverse of a heterolysis reaction. |
|
| Coordination To A Metal Ion |

|
| A species coordinates to a metal ion during the course of this reaction. |
|
| Cyclisation |

|
| The formation of a ring compound from a chain by the formation of a new bond. |
|
| Deamination |

|
| Ammonia is one of the products of the reaction. It may be produced either through elimination, or some other mechanism. |
|
| Decarboxylation |

|
| A reaction in which carbon dioxide is eliminated from one of the reacting species. |
|
| Decoordination From A Metal Ion |

|
| A species de-coordinates from a metal ion during the course of this reaction. |
|
| Decyclisation |

|
| The formation of a chain compound from a ring by the cleavage of an existing bond. |
|
| Dehydration |

|
| A reaction in which water is one of the products of the reaction. |
|
| Dephosphorylation |

|
| A reaction during which a phosphate moiety is a product of the reaction. This can be anything from a monophosphate to a triphosphate. |
|
| Electron Relay |

|
| A mechanism component which states that the associated reaction contains an electron relay, i.e. a chemical species that both accepts and donates electrons during the course of the reaction. |
|
| Electron Transfer |

|
| A reaction in which an electron is transferred from one reacting species to another. |
|
| Elimination (Not Covered By The Ingold Mechanisms) |

|
| In an elimination, one or more groups are lost, most often from two different centres with concomitant formation of an unsaturation in the molecule (double bond, triple bond) or the formation of a new ring. When the term elimination is used in its own, it refers to an elimination reaction not covered by the standard CK Ingold reaction mechanisms. |
|
| Enzyme-Substrate Complex Cleavage |

|
| One or more covalent bonds between the enzyme and substrate are cleaved, either heterolytically or homolytically, during the associated reaction. |
|
| Enzyme-Substrate Complex Formation |

|
| One or more covalent bonds between the enzyme and substrate are formed, either heterolytically or homolytically, during the associated reaction. |
|
| Heterolysis |

|
| The cleavage of a covalent bond so that both bonding electrons remain with one of the two fragments between which the bond is broken. This is the reverse of a coordination reaction. |
|
| Homolysis |

|
| The cleavage of a bond so that each of the molecular fragments between which the bond is broken retains one of the bonding electrons. This is the reverse of a colligation reaction. |
|
| Hydride Relay |

|
| The reaction contains a hydride relay, i.e. a chemical species that both accepts and donates a hydride ion during the course of the reaction. |
|
| Hydride Transfer |

|
| A reaction in which a hydride anion is transferred from one reacting species to another. |
|
| Hydrogen Relay |

|
| This reaction contains a hydrogen relay, i.e. a chemical species that acts as both hydrogen acceptor and hydrogen donor during the course of the reaction. |
|
| Hydrogen Transfer |

|
| A reaction in which a hydrogen atom is transferred from one reacting species to another. |
|
| Hydrolysis |

|
| A reaction during which nucleophilic attack by water leads to the cleavage of one or more bonds in a reactant. |
|
| Inferred Reaction Step |

|
| A reaction step which has been inferred from the literature entries. These steps normally consist only of "obvious" proton transfers and have been added in order to return the enzymes to their resting states (inferred return steps). There are also reactions such as transaldiminations and Schiff base formations which are commonly not cited as a stepwise reaction in the literature references, and we have had to infer their step-by-step mechanisms. |
|
| Intermediate Collapse |

|
| A reaction in which an intermediate collapses to form two or more species, which may be a product or another intermediate. |
|
| Intermediate Formation |

|
| A reaction in which an intermediate is formed. |
|
| Intermediate Terminated |

|
| A reaction in which an intermediate only forms a product of the overall reaction. |
|
| Intramolecular Rearrangement |

|
| The term is traditionally applied to any reaction that involves a change of connectivity (sometimes including hydrogen), and violates the so-called principle of minimum structural change. |
|
| Isomerisation Reaction (Not Covered By Named Reactions) |

|
| A reaction in which the principal product is isomeric with the principal reactant. |
|
| Keto-Enol Tautomerisation |

|
| Tautomerisation of the form: H-C-C=O <=> C=C-O-H and is not assisted by external proton transfers. |
|
| Michael Addition |

|
| 1,4-nucleophilic addition. Occurs on addition of a "soft" nucleophile to an alpha-beta unsaturated carbonyl compound. |
|
| Native State Of Cofactor Is Not Regenerated |

|
| The enzyme's cofactor is left in an inactive state. This should only be used if the cofactor is not regenerated at the end of the mechanism. |
|
| Native State Of Cofactor Regenerated |

|
| A mechanism component which states that during the course of the associated reaction a molecule listed as a cofactor (in the overall reaction) is returned to a state in which it is able to undergo another round of catalysis. |
|
| Native State Of Enzyme Is Not Regenerated |

|
| The enzyme is not returned to its ground state, or a state in which it is ready to perform another catalytic cycle, by the end of this reaction. This term should only be applied to the final step in which a change occurs within the enzyme. |
|
| Native State Of Enzyme Regenerated |

|
| The enzyme is returned to its ground state, or a state in which it is ready to perform another catalytic cycle, by the end of this reaction. This term should only be applied to the final step in which a change occurs in the enzyme. |
|
| Overall Product Formed |

|
| An overall product of the enzyme reaction is produced in this step. The step may be producing either the overall product itself, or the result of the enzymatic reaction, which then undergoes some spontaneous change, not necessarily occurring within the enzyme, to form one of the overall products. |
|
| Overall Reactant Used |

|
| An overall reactant of the enzyme is consumed in this step. The step may be using either the overall reactant itself or the result of some spontaneous change on one of the overall reactants which occurs just prior to the enzymatic reaction. |
|
| Pericyclic Reaction |

|
| Chemical reaction in which concerted reorganisation of bonding takes place throughout a cyclic array of continuously bonded atoms. An example of a pericyclic reaction is the Claisen rearrangement. |
|
| Photochemical Activation |

|
| The activation of a chemical species by light. |
|
| Proton Relay |

|
| The reaction contains a proton relay, i.e. a chemical species that acts as both Brønsted base and Brønsted acid during the course of the reaction. |
|
| Proton Transfer |

|
| A reaction in which a proton is transferred from one reacting species to another. |
|
| Radical Formation |

|
| A reaction in which the initial generation of free radicals occurs. |
|
| Radical Propagation |

|
| A reaction in which the free radical causes some chemical change, but it is not lost. |
|
| Radical Termination |

|
| A reaction in which the free radical is lost. |
|
| Rate-Determining Step |

|
| This is the step in a reaction which has the slowest rate, thus imposing an upper limit on the overall reaction rate. |
|
| Reaction Occurs Outside The Enzyme |

|
| A reaction which occurs outside of the enzyme and that is generally spontaneous. These steps are included in MACiE because they either spontaneously form the enzyme's substrate from the overall reactants of the reaction as defined by EC or form the overall products of the EC reaction from an intermediate generated by the enzyme. |
|
| Redox Reaction |

|
| An oxidation-reduction (redox) reaction is a type of chemical reaction that involves a transfer of electrons between two species. Any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron. |
|
| Schiff Base Formed |

|
| A mechanism component which states that during the course of the associated reaction there is a formation of an imine bearing a hydrocarbyl group on the nitrogen atom. The mechanism of forming Schiff bases is also known as a Schiff condensation. This is a multi-step reaction and as such we only term the final step the actual formation of the Schiff base. |
|
| Sigmatropic Rearrangement |
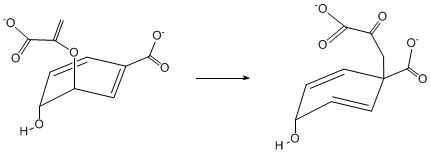
|
| Molecular rearrangement involving both the creation of a new σ-bond between atoms previously not directly linked and the breaking of an existing sigma-bond. Often accompanied by a concurrent relocation of pi-bonds in the molecule, however the total number of pi- and sigma- bonds remains the same. |
|
| Substitution (Not Covered By The Ingold Mechanisms) |

|
| A reaction, either elementary or stepwise, in which one atom or group in a molecular entity is replaced by another atom or group. In addition to the substitution reactions already defined in MACiE, there also exist the acidic SN2, acidic SNi, allylic SN2 and allylic SNi. When the term substitution is used in its own, it refers to a substitution reaction not covered by the above Ingold reaction mechanisms. |
|
| Tautomerisation (Not Keto-Enol) |

|
| Isomerism of the general form GXY=Z <=> X=YZG where the isomers are readily inter-convertible; the atoms connecting the groups X,Y,Z are typically any of C, H, O or S, and G is a group which becomes an electrofuge or nucleofuge during isomerisation. This term is used for any tautomerisation (which is not assisted by external general acid/base residues) that is not of the keto-enol form. |
|
C. K. Ingold Mechanisms
Although the following mechanisms are all included in the components list, this section gives a more detailed description of the C. K. Ingold treatment of chemical mechanisms. This leads to a rigorous treatment of the Ingold mechanisms lead to a precisely defined set of possibilities. Following are the possible Ingold Mechanisms allowed for in MACiE. It should be noted that whilst we allow for unimolecular mechanisms, due to the fact that we classify reactions on a per-step basis these will only be assigned in exceptional circumstances.
C. K. Ingold was the first to attempt a systematic representation of reactions in the 1930's. His mechanistic nomenclature (SN2, E1, etc.) and notational tool, the "curly arrows" or "arrow pushing", are now familiar to the modern day chemist. He defined a chemical reaction as an electrical transaction, which takes place by virtue of some predominating constitutional affinity either for atomic nuclei or electrons on the part of a reagent; or perhaps for both, i.e. the atoms themselves1. He clusters reagents with an even number of electrons (basically nucleophiles and electrophiles) into the first and second classes and reagents with an odd number of electrons (basically radicals) into the third, rarer, category.
Ingold split his reactions firstly into the categories of homolytic and heterolytic. Homolytic reactions are those reactions in which covalent bond formation (colligation) is by the supply of one electron from each of the reacting species. The reverse of this is homolysis, where the bonding electrons are split equally between the two atoms involved in the bond. Heterolytic reactions are those in which one reacting species supplies both electrons or, in the reverse reaction, receives both electrons. Thus heterolytic reactions can be either nucleophilic or electrophilic, whilst homolytic reactions are simply homolytic. Ingold defines nucleophiles as those reagents that act by donating their electrons, or sharing them with, a foreign atomic nucleus, and electrophiles as those reagents which act by acquiring electrons, or a share in electrons, which had previously belonged exclusively to a foreign molecule.
From this, the reaction can be defined as an addition, substitution or elimination. Unfortunately, there are very few rules as to which species is designated the attacking species and so it falls to pure convention as to whether the reaction is nucleophilic or electrophilic. It is suggested by Ingold that in order to assign a substitution reaction the actual mechanism is not necessary as it is obvious as to the identity of the substitution species:
OET- + CH3I --> CH3OEt + I-
the iodide is obviously being substituted for the ethoxide, hence the reaction is considered to be nucleophilic and termed SN.
NO2+ + Ph-H --> PhNO2 + H+
The nitro group is obviously displacing the proton, hence the reaction is considered to be electrophilic and termed SE. A homolytic substitution is thus termed an SH reaction.
Addition reactions are more difficult as the mechanism becomes more important. However, with the application of chemical knowledge it is not as difficult as it might at first seem:
NH3 + CH3CH=O --> CH3CH(OH)NH2
here the mechanism is nucleophilic in nature and thus it is termed an AdN.
H-Cl + CH3CH=CH2 --> CH3CHClCH3
here the mechanism is electrophilic, with the p-electrons in the double bond attacking the H in H-Cl, thus it is termed an AdE. A homolytic addition is termed an AdH reaction.
Ingold states that all elimination reactions are nucleophilic in nature and as such it is not necessary to term them EN, simply E. However, in MACiE we have a case of homolytic elimination and so we have added the term EH to our definitions.
Ingold adds that there are reactions not treated under the general rules discussed above, and included in these reactions are rearrangements. Rearrangements are described according to their reaction type, i.e. nucleophilic, electrophilic and homolytic.
Finally, reactions can be unimolecular, i.e. the rate determining step only involves one species, bimolecular, i.e. the rate determining step involved two species, or intramolecular, i.e. the rate determining step involves two centres reacting with each other which are separate in space, but belong to the same molecule. It is believed that all reactions can thus be assigned an Ingold Mechanism.
The following are Ingold mechanisms allowed for in MACiE:
Additions
A chemical reaction of two or more reacting molecular entities, resulting in a single reaction product containing all atoms of all components, with formation of at least one chemical bond and a net reduction in bond multiplicity in at least one of the reactants. The following addition reactions are defined in the MACiE dictionary:
|
Nucleophilic |
Electrophilic |
Homolytic |
| Bimolecular |
 |
 |
 |
| Aromatic Bimolecular |
 |
 |
X |
| Intramolecular |
 |
 |
 |
If there is no obviously assignable Ingold mechanism, addition may then be used as a reaction type. It is suggested that addition only be used in these circumstances and requires no further input.
The following are examples of addition reactions in MACiE:
| Aromatic Bimolecular Electrophilic Addition |
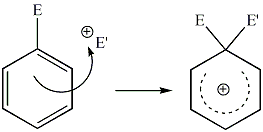
|
| A bimolecular electrophilic addition to an aromatic species. This may also be viewed as the first step of an electrophilic aromatic substitution. |
|
| Bimolecular Electrophilic Addition |
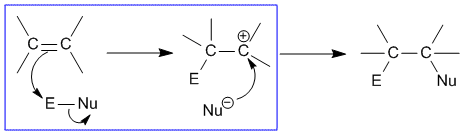
|
| An addition of an electrophilic species over a pi-bond of another species. The reaction involves the collision of two species in its rate determining step. |
|
| Intramolecular Electrophilic Addition |

|
| An addition of an electrophilic moiety of a species over a π-bond of the same species. The reaction proceeds via a cyclic transition state. |
|
| Bimolecular Homolytic Addition |

|
| An addition of a radical species over a π-bond of another species. The reaction involves the collision of two species in its rate determining step. |
|
| Intramolecular Homolytic Addition |

|
| An addition of a radical moiety of a species over a π-bond of the same species. The reaction proceeds via a cyclic transition state. |
|
| Aromatic Bimolecular Nucleophilic Addition |

|
| A bimolecular nucleophilic addition to an aromatic species. This may also be viewed as the first step of a nucleophilic aromatic substitution. |
|
| Bimolecular Nucleophilic Addition |

|
| An addition of a nucleophilic species over a π-bond of another species. The reaction involves the collision of two species in its rate determining step. |
|
| Intramolecular Nucleophilic Addition |

|
| An addition of a nucleophilic moiety of a species over a π-bond of the same species. The reaction proceeds via a cyclic transition state. |
|
Elimination
In an elimination one or more groups are lost, most often from two different centres, with concomitant formation of an unsaturation in the molecule (double bond, triple bond) or formation of a new ring. The following types of elimination are allowed in MACiE and correspond to the Ingold elimination reaction mechanisms.
|
| Nucleophilic |
Homolytic |
| Bimolecular |
 |
 |
| Aromatic Bimolecular |
 |
X |
| Intramolecular |
 |
 |
| Aromatic Intramolecular |
 |
X |
| Unimolecular |
 |
 |
| Aromatic Unimolecular |
 |
X |
If there is no obviously assignable Ingold mechanism, elimination may then be used as a reaction type. It is suggested that elimination only be used in these circumstances and requires no further input.
The following are examples of elimination reactions in MACiE:
| Bimolecular Homolytic Elimination |

|
| A homolytic elimination reaction which proceeds via second order kinetics and involves free radical chemistry. The rate determining step involves two chemical species undergoing covalency changes. |
|
| Intramolecular Homolytic Elimination |

|
| A homolytic elimination reaction in which all the components are in the same chemical species. The reaction proceeds via a cyclic transition state. |
|
| Unimolecular Homolytic Elimination |

|
| A homolytic unimolecular elimination reaction in which a radical moiety eliminates another radical moiety from the same species by forming a double bond (or cyclic compound). The rate determining step involves a single species. |
|
| Aromatic Bimolecular Elimination |

|
| A bimolecular elimination from an aromatic species. |
|
| Bimolecular Elimination |

|
| A nucleophilic elimination reaction which proceeds via second order kinetics. The rate determining step involves two chemical species undergoing covalency changes. |
|
| Aromatic Intramolecular Elimination |

|
| An intramolecular elimination reaction from an aromatic species. |
|
| Intramolecular Elimination |

|
| A nucleophilic elimination reaction in which all the components are in the same chemical species. The reaction proceeds via a cyclic transition state. |
|
| Aromatic Unimolecular Elimination By The Conjugate Base |

|
| An unimolecular elimination by the conjugate base from an aromatic species. |
|
| Unimolecular Elimination By The Conjugate Base |

|
| A unimolecular elimination reaction in which a conjugate base species eliminates an atom or group from itself to form a double bond (or cyclic compound). |
|
Substitutions
A reaction, either elementary or stepwise, in which one atom or group in a molecular entity is replaced by another atom or group. The following substitution reactions are defined in the MACiE dictionary:
|
Nucleophilic |
Electrophilic |
Homolytic |
| Bimolecular |
 |
 |
 |
| Aromatic Bimolecular |
 |
X |
X |
| Intramolecular |
 |
 |
 |
In addition to the above types of substitutions, there also exist the acidic SN2, acidic SNi, allylic SN2 and allylic SNi.
If there is no obviously assignable Ingold mechanism, substitution may then be used as a reaction type. It is suggested that substitution only be used in these circumstances and requires no further input.
The following are examples of substitution reactions in MACiE:
| Bimolecular Electrophilic Substitution |
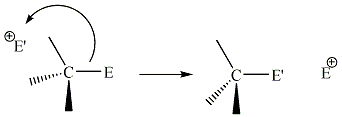
|
| An electrophilic substitution which proceeds with second order kinetics i.e. the rate determining step of the reaction involves the collision of two chemical species. |
|
| Aromatic Intramolecular Electrophilic Substitution |

|
| An electrophilic moiety in an aromatic species substitutes another electrophilic moiety from the same species. The reaction proceeds via a cyclic transition state. |
|
| Intramolecular Electrophilic Substitution |

|
| An electrophilic moiety in a species substitutes another electrophilic moiety from the same species. The reaction proceeds via a cyclic transition state. |
|
| Bimolecular Homolytic Substitution |

|
| A homolytic substitution which proceeds with second order kinetics i.e. the rate determining step of the reaction involves the collision of two chemical species. |
|
| Intramolecular Homolytic Substitution |

|
| A radical moiety in a species substitutes another radical moiety from the same species. The reaction proceeds via a cyclic transition state. |
|
| Acidic Bimolecular Nucleophilic Substitution |

|
| This is a bimolecular nucleophilic substitution, in which the leaving group is protonated prior to the substitution event. The primed group, e.g. a hydroxide group, would otherwise be a poor leaving group. Thus, by protonation, it is made into a much better leaving group. The rate determining step of the reaction involves the collision of two species. In the figure below, the actual substitution is shown enclosed in the blue box. |
|
| Aromatic Bimolecular Nucleophilic Substitution |

|
| A bimolecular nucleophilic substitution upon an aromatic system. It can occur as a two step process: addition of a nucleophilic reagent upon the aromatic ring to form a carbanion, followed by elimination of a nucleofuge, or vice versa, or can occur as a one step process with nucleophilic attack and nucleofuge elimination happening simultaneously. |
|
| Bimolecular Nucleophilic Substitution |

|
| A nucleophilic substitution which proceeds with second order kintetics, i.e. the rate determining step of the reaction involves the collision of two chemical species. |
|
| Bimolecular Nucleophilic Substitution With Allylic Rearrangement |

|
| This is a bimolecular nucleophilic substitution reaction with concurrent allylic rearrangement of the double bonds in a conjugated system. |
|
| Acidic Intramolecular Nucleophilic Substitution |

|
| This is a intramolecular nucleophilic substitution, in which the leaving group is protonated prior to the substitution event. The primed group, e.g. a hydroxide group, would otherwise be a poor leaving group. Thus, by protonation, it is made into a much better leaving group. The rate determining step of the reaction involves a single species and proceeds via a cyclic transition state. |
|
| Intramolecular Nucleophilic Substitution |

|
| A nucleophilic moiety in a species substitutes another nucleophilic moiety from the same species. The reaction proceeds via a cyclic transition state. |
|
| Intramolecular Nucleophilic Substitution With Allylic Rearrangement |

|
| This is an intramolecular nucleophilic substitution reaction with concurrent allylic rearrangement of the double bonds in a conjugated system. |
|
References
- C. K. Ingold, Structure and Mechanism in Organic Chemistry, 2nd Ed, Cornell University Press, Ithaca, N.Y., Chapters 5-15, (1969).


























































