 |
PDBsum entry 4z9a
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.3.1.3.2
- acid phosphatase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a phosphate monoester + H2O = an alcohol + phosphate
|
 |
 |
 |
 |
 |
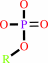 phosphate monoester
phosphate monoester
|
+
|
H2O
|
=
|
 alcohol
alcohol
|
+
|
 phosphate
phosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.3.1.3.48
- protein-tyrosine-phosphatase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
O-phospho-L-tyrosyl-[protein] + H2O = L-tyrosyl-[protein] + phosphate
|
 |
 |
 |
 |
 |
O-phospho-L-tyrosyl-[protein]
|
+
|
H2O
|
=
|
L-tyrosyl-[protein]
|
+
|
 phosphate
phosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Bioorg Med Chem Lett
23:4462-4471
(2015)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structures of the apo form and a complex of human LMW-PTP with a phosphonic acid provide new evidence of a secondary site potentially related to the anchorage of natural substrates.
|
|
E.M.Fonseca,
D.B.Trivella,
V.Scorsato,
M.P.Dias,
N.L.Bazzo,
K.R.Mandapati,
F.L.de Oliveira,
C.V.Ferreira-Halder,
R.A.Pilli,
P.C.Miranda,
R.Aparicio.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Low molecular weight protein tyrosine phosphatases (LMW-PTP, EC 3.1.3.48) are a
family of single-domain enzymes with molecular weight up to 18kDa, expressed in
different tissues and considered attractive pharmacological targets for cancer
chemotherapy. Despite this, few LMW-PTP inhibitors have been described to date,
and the structural information on LMW-PTP druggable binding sites is scarce. In
this study, a small series of phosphonic acids were designed based on a new
crystallographic structure of LMW-PTP complexed with benzylsulfonic acid,
determined at 2.1Å. In silico docking was used as a tool to interpret the
structural and enzyme kinetics data, as well as to design new analogs. From the
synthesized series, two compounds were found to act as competitive inhibitors,
with inhibition constants of 0.124 and 0.047mM. We also report the 2.4Å
structure of another complex in which LMW-PTP is bound to benzylphosphonic acid,
and a structure of apo LMW-PTP determined at 2.3Å resolution. Although no
appreciable conformation changes were observed, in the latter structures, amino
acid residues from an expression tag were found bound to a hydrophobic region at
the protein surface. This regions is neighbored by positively charged residues,
adjacent to the active site pocket, suggesting that this region might be not a
mere artefact of crystal contacts but an indication of a possible anchoring
region for the natural substrate-which is a phosphorylated protein.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

