
|

|

|
|
 |
Contents |
 |
|
|

|

|

|

|

|

|

|





 (+ 5 more)
292 a.a.
(+ 5 more)
292 a.a.
|
 |

|

|

|

|

|

|

|
 274 a.a.
274 a.a.
|
 |

|

|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
Arginine feed-back inhibitable acetylglutamate kinase
|
|
Structure:
|
 |
Acetylglutamate kinase. Chain: a, b, c, d, e, f, g, h, i, j, k, l. Synonym: NAG kinase, agk, n-acetyl-l-glutamate 5-phosphotransferase. Engineered: yes
|
|
Source:
|
 |
Pseudomonas aeruginosa. Organism_taxid: 287. Expressed in: escherichia coli. Expression_system_taxid: 469008.
|
|
Biol. unit:
|
 |
 Hexamer (from PDB file)
Hexamer (from PDB file)
|
|
Resolution:
|
 |
|
2.95Å
|
R-factor:
|
0.249
|
R-free:
|
0.267
|
|
|
Authors:
|
 |
S.Ramon-Maiques,M.L.Fernandez-Murga,A.Vagin,I.Fita,V.Rubio
|
Key ref:

|
 |
S.Ramón-Maiques
et al.
(2006).
Structural Bases of Feed-back Control of Arginine Biosynthesis, Revealed by the Structures of Two Hexameric N-Acetylglutamate Kinases, from Thermotoga maritima and Pseudomonas aeruginosa.
J Mol Biol,
356,
695-713.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
12-Jun-05
|
Release date:
|
13-Dec-05
|
|
|
Supersedes:
|

|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B, C, D, E, F, G, H, I, J, K, L:
E.C.2.7.2.8
- acetylglutamate kinase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Ornithine Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
N-acetyl-L-glutamate + ATP = N-acetyl-L-glutamyl 5-phosphate + ADP
|
 |
 |
 |
 |
 |
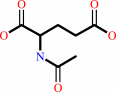 N-acetyl-L-glutamate
N-acetyl-L-glutamate
|
+
|
ATP
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
N-acetyl-L-glutamyl 5-phosphate
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
 ADP
ADP
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Mol Biol
356:695-713
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural Bases of Feed-back Control of Arginine Biosynthesis, Revealed by the Structures of Two Hexameric N-Acetylglutamate Kinases, from Thermotoga maritima and Pseudomonas aeruginosa.
|
|
S.Ramón-Maiques,
M.L.Fernández-Murga,
F.Gil-Ortiz,
A.Vagin,
I.Fita,
V.Rubio.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
N-Acetylglutamate kinase (NAGK) catalyses the second step in the route of
arginine biosynthesis. In many organisms this enzyme is inhibited by the final
product of the route, arginine, and thus plays a central regulatory role. In
addition, in photosynthetic organisms NAGK is the target of the
nitrogen-signalling protein P(II). The 3-D structure of homodimeric,
arginine-insensitive, Escherichia coli NAGK, clarified substrate binding and
catalysis but shed no light on arginine inhibition of NAGK. We now shed light on
arginine inhibition by determining the crystal structures, at 2.75A and 2.95A
resolution, of arginine-complexed Thermotoga maritima and arginine-free
Pseudomonas aeruginosa NAGKs, respectively. Both enzymes are highly similar
ring-like hexamers having a central orifice of approximately 30A diameter. They
are formed by linking three E.coli NAGK-like homodimers through the interlacing
of an N-terminal mobile kinked alpha-helix, which is absent from E.coli NAGK.
Arginine is bound in each subunit of T.maritima NAGK, flanking the interdimeric
junction, in a site formed between the N helix and the C lobe of the subunit.
This site is also present, in variable conformations, in P.aeruginosa NAGK, but
is missing from E.coli NAGK. Arginine, by gluing the C lobe of each subunit to
the inter-dimeric junction, may stabilize an enlarged active centre
conformation, hampering catalysis. Acetylglutamate counters arginine inhibition
by promoting active centre closure. The hexameric architecture justifies the
observed sigmoidal arginine inhibition kinetics with a high Hill coefficient (N
approximately 4), and appears essential for arginine inhibition and for
NAGK-P(II) complex formation, since this complex may involve binding of NAGK and
P(II) with their 3-fold axes aligned. The NAGK structures allow identification
of diagnostic sequence signatures for arginine inhibition. These signatures are
found also in the homologous arginine-inhibited enzyme NAG synthase. The
findings on NAGK shed light on the structure, function and arginine inhibition
of this synthase, for which a hexameric model is constructed.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Figure 2. Architecture of arginine-sensitive NAGK. The
TmNAGK ((a) and (b)) or PaNAGK ((e) and (f)) hexamers are viewed
((a) and (e)) along or ((b) and (f)) perpendicularly to the
molecular 3-fold axis. Each homodimer is coloured differently.
In (a) and (b) arginine, and in (e) and (f) MgADP and NAG are
represented in space-filling representation and coloured. In (a)
and (e) the arrowpoints indicate the interlaced N helices at one
junction. (c) TmNAGK and (d) EcNAGK26 homodimers, viewed along
their 2-fold axes. The N and C lobes are in blue and green,
respectively, and the N helix is in red. In (c) arginine is
shown in space-filling representation, and coloured. The ligands
shown in (d) are in ball and stick representation, and are NAG
and AMPPNP.
|
 |
Figure 3.
Figure 3. Amino acid sequence and topology of secondary
structure elements, and signature sequences of
arginine-sensitive NAGK. (a) Sequence alignment of E. coli, P.
aeruginosa and T. maritima NAGKs (Swissprot P0A6C8, Q9HTN2 and
Q9X2A4, respectively), localizing the secondary structure
elements as superimposed blue arrows (b-strands), and yellow
(a-helices) or orange (N-terminal helix) rectangles. The
residues conserved or conservatively replaced in all NAGKs are
in red, those having decreased accessibility upon the binding of
NAG, MgADP or arginine are indicated with dark green triangles,
light green triangles and violet diamonds, respectively. Black
and grey circles denote decreased accessibility upon homodimer
and hexamer formation, respectively. Signature sequence traits
associated with arginine inhibition are underlined. (b) Scheme
of the topology of secondary structure elements found in NAGKs,
where b-strands and a-helices are represented as triangles and
circles, respectively, the strands of the central b-sheet are
shadowed, and the colour code is red for the N helix (the only
element missing in EcNAGK; represented as two circles because of
the kink), and green and blue for the elements of the N and the
C lobe, respectively. (c) Alignment (see Materials and Methods)
of arginine-insensitive and arginine-sensitive NAGKs in the
three regions (separated by vertical lines) where diagnostic
signatures were identified. Residues found constantly and
exclusively in arginine-sensitive NAGKs are highlighted in red.
The K/R highlighted in blue is found constantly but not
exclusively, in arginine-sensitive NAGKs. The residues
highlighted in pink are exclusively (but not constantly) found
in arginine-sensitive NAGKs. Yellow colouring highlights
residues that are conserved or conservatively replaced in most
NAGKs, irrespective of whether they are sensitive or insensitive
to arginine. Rectangles and arrows above the alignment indicate,
respectively, a-helices and b-strands, as they appear in PaNAGK.
The horizontal line below the alignment marks the larger (see
the text) sequence signature at the b15-aH-b16 region. A
rectangle encloses the phenylalanine residues of yeast and
Neurospora crassa NAGKs that when mutated resulted in hampered
arginine inhibition.16 The abbreviations used and the
Swissprot/Trembl (unless indicated otherwise) accession numbers
(given between parentheses) are the following: ECOLI, E. coli
(P0A6C8); SERMA, S. marcescens (encoded by nucleotides
4275578-4274805 of the S. marcescens genome,
systematic_id=SMA4004,
http://www.sanger.ac.uk/projects/s_marcescens/sma.art); BACSU,
B. subtilis (P68729); BACST, Bacillus stearothermophilus
(Q07905); PSEAE, P. aeruginosa (Q9HTN2); THEMA, T. maritima
(Q9X2A4); CORGL, Corynebacterium glutamicum;13 SYNP7, S.
elongatus, strain PCC7942 (Q6V1L5). The sequences of
photosynthetic eukaryotes start after a predicted chloroplast
signal targeting sequence that precedes the N-terminal
extension: CREIN, Chlamydomonas reinhardtii (gene TC25068,
http://www.tigr.org/tdb/tgi/chrgi); ORYSA, Oriza sativa (rice,
Q949B4); ARATH: Arabidopsis thaliana (Q8LA25); the rice and A.
thaliana NAGKs are assumed to be arginine-sensitive by
similarity to the pea enzyme18 (for which no sequence is
available) and also because both are known to interact with the
nitrogen signalling protein P[II].19^ and 21 The fungal
sequences start after the mitochondrial signal targeting
sequence that precedes the N-terminal extension: YEAST,
Saccharomyces cerevisiae (Q01217); NEUCR, N. crassa (P54898).
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Elsevier:
J Mol Biol
(2006,
356,
695-713)
copyright 2006.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
E.Marcos,
R.Crehuet,
and
I.Bahar
(2010).
On the conservation of the slow conformational dynamics within the amino acid kinase family: NAGK the paradigm.
|
| |
PLoS Comput Biol,
6,
e1000738.
|
 |
|
|
|
|
 |
I.Pérez-Arellano,
and
J.Cervera
(2010).
Glutamate kinase from Thermotoga maritima: characterization of a thermophilic enzyme for proline biosynthesis.
|
| |
Extremophiles,
14,
409-415.
|
 |
|
|
|
|
 |
L.Caldovic,
N.Ah Mew,
D.Shi,
H.Morizono,
M.Yudkoff,
and
M.Tuchman
(2010).
N-acetylglutamate synthase: structure, function and defects.
|
| |
Mol Genet Metab,
100,
S13-S19.
|
 |
|
|
|
|
 |
N.Dellas,
and
J.P.Noel
(2010).
Mutation of archaeal isopentenyl phosphate kinase highlights mechanism and guides phosphorylation of additional isoprenoid monophosphates.
|
| |
ACS Chem Biol,
5,
589-601.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.Min,
Z.Jin,
L.Caldovic,
H.Morizono,
N.M.Allewell,
M.Tuchman,
and
D.Shi
(2009).
Mechanism of Allosteric Inhibition of N-Acetyl-L-glutamate Synthase by L-Arginine.
|
| |
J Biol Chem,
284,
4873-4880.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.S.Kalamaki,
D.Alexandrou,
D.Lazari,
G.Merkouropoulos,
V.Fotopoulos,
I.Pateraki,
A.Aggelis,
A.Carrillo-López,
M.J.Rubio-Cabetas,
and
A.K.Kanellis
(2009).
Over-expression of a tomato N-acetyl-L-glutamate synthase gene (SlNAGS1) in Arabidopsis thaliana results in high ornithine levels and increased tolerance in salt and drought stresses.
|
| |
J Exp Bot,
60,
1859-1871.
|
 |
|
|
|
|
 |
D.Shi,
V.Sagar,
Z.Jin,
X.Yu,
L.Caldovic,
H.Morizono,
N.M.Allewell,
and
M.Tuchman
(2008).
The crystal structure of N-acetyl-L-glutamate synthase from Neisseria gonorrhoeae provides insights into mechanisms of catalysis and regulation.
|
| |
J Biol Chem,
283,
7176-7184.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.L.Llácer,
I.Fita,
and
V.Rubio
(2008).
Arginine and nitrogen storage.
|
| |
Curr Opin Struct Biol,
18,
673-681.
|
 |
|
|
|
|
 |
M.L.Fernández-Murga,
and
V.Rubio
(2008).
Basis of arginine sensitivity of microbial N-acetyl-L-glutamate kinases: mutagenesis and protein engineering study with the Pseudomonas aeruginosa and Escherichia coli enzymes.
|
| |
J Bacteriol,
190,
3018-3025.
|
 |
|
|
|
|
 |
N.Haskins,
M.Panglao,
Q.Qu,
H.Majumdar,
J.Cabrera-Luque,
H.Morizono,
M.Tuchman,
and
L.Caldovic
(2008).
Inversion of allosteric effect of arginine on N-acetylglutamate synthase, a molecular marker for evolution of tetrapods.
|
| |
BMC Biochem,
9,
24.
|
 |
|
|
|
|
 |
S.Pakhomova,
S.G.Bartlett,
A.Augustus,
T.Kuzuyama,
and
M.E.Newcomer
(2008).
Crystal Structure of Fosfomycin Resistance Kinase FomA from Streptomyces wedmorensis.
|
| |
J Biol Chem,
283,
28518-28526.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.L.Llácer,
A.Contreras,
K.Forchhammer,
C.Marco-Marín,
F.Gil-Ortiz,
R.Maldonado,
I.Fita,
and
V.Rubio
(2007).
The crystal structure of the complex of PII and acetylglutamate kinase reveals how PII controls the storage of nitrogen as arginine.
|
| |
Proc Natl Acad Sci U S A,
104,
17644-17649.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
Q.Qu,
H.Morizono,
D.Shi,
M.Tuchman,
and
L.Caldovic
(2007).
A novel bifunctional N-acetylglutamate synthase-kinase from Xanthomonas campestris that is closely related to mammalian N-acetylglutamate synthase.
|
| |
BMC Biochem,
8,
4.
|
 |
|
|
|
|
 |
Y.Mizuno,
G.B.Moorhead,
and
K.K.Ng
(2007).
Structural basis for the regulation of N-acetylglutamate kinase by PII in Arabidopsis thaliana.
|
| |
J Biol Chem,
282,
35733-35740.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
Y.Xu,
B.Labedan,
and
N.Glansdorff
(2007).
Surprising arginine biosynthesis: a reappraisal of the enzymology and evolution of the pathway in microorganisms.
|
| |
Microbiol Mol Biol Rev,
71,
36-47.
|
 |
|
|
|
|
 |
D.Shi,
L.Caldovic,
Z.Jin,
X.Yu,
Q.Qu,
L.Roth,
H.Morizono,
Y.Hathout,
N.M.Allewell,
and
M.Tuchman
(2006).
Expression, crystallization and preliminary crystallographic studies of a novel bifunctional N-acetylglutamate synthase/kinase from Xanthomonas campestris homologous to vertebrate N-acetylglutamate synthase.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
62,
1218-1222.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

