 |
PDBsum entry 1w4x

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.14.13.92
- phenylacetone monooxygenase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
phenylacetone + NADPH + O2 + H+ = benzyl acetate + NADP+ + H2O
|
 |
 |
 |
 |
 |
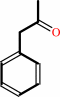 phenylacetone
phenylacetone
|
+
|
 NADPH
NADPH
|
+
|
 O2
O2
|
+
|
H(+)
|
=
|
 benzyl acetate
benzyl acetate
|
+
|
 NADP(+)
NADP(+)
|
+
|
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FAD
|
 |
 |
 |
 |
 |
FAD
Bound ligand (Het Group name =
FAD)
corresponds exactly
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Proc Natl Acad Sci U S A
101:13157-13162
(2004)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structure of a Baeyer-Villiger monooxygenase.
|
|
E.Malito,
A.Alfieri,
M.W.Fraaije,
A.Mattevi.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Flavin-containing Baeyer-Villiger monooxygenases employ NADPH and molecular
oxygen to catalyze the insertion of an oxygen atom into a carbon-carbon bond of
a carbonylic substrate. These enzymes can potentially be exploited in a variety
of biocatalytic applications given the wide use of Baeyer-Villiger reactions in
synthetic organic chemistry. The catalytic activity of these enzymes involves
the formation of two crucial intermediates: a flavin peroxide generated by the
reaction of the reduced flavin with molecular oxygen and the "Criegee"
intermediate resulting from the attack of the flavin peroxide onto the substrate
that is being oxygenated. The crystal structure of phenylacetone monooxygenase,
a Baeyer-Villiger monooxygenase from the thermophilic bacterium Thermobifida
fusca, exhibits a two-domain architecture resembling that of the disulfide
oxidoreductases. The active site is located in a cleft at the domain interface.
An arginine residue lays above the flavin ring in a position suited to stabilize
the negatively charged flavin-peroxide and Criegee intermediates. This amino
acid residue is predicted to exist in two positions; the "IN" position
found in the crystal structure and an "OUT" position that allows NADPH
to approach the flavin to reduce the cofactor. Domain rotations are proposed to
bring about the conformational changes involved in catalysis. The structural
studies highlight the functional complexity of this class of flavoenzymes, which
coordinate the binding of three substrates (molecular oxygen, NADPH, and
phenylacetone) in proximity of the flavin cofactor with formation of two
distinct catalytic intermediates.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Fig. 1. Schematic representation of the overall catalytic
reaction of the Baeyer-Villiger monooxygenases with reference to
PAMO (mainly based on the kinetic analysis by Sheng et al. (7)
of cyclohexanone monooxygenase). The atomic numbering of the
flavin ring is shown on the left (corresponding to the initial
step of the reaction).
|
 |
Figure 4.
Fig. 4. Stereo view of the flavin-binding site. The
orientation is approximately the same as that of Fig. 3. The
flavin has a planar conformation although its N10 atom exhibits
a considerable degree of pyramidalization, which positions the
C1 atom of the ribityl chain out of the flavin plane. Carbons
are shown in black, oxygens are shown in red, and nitrogens are
shown in blue. Ordered water molecules are shown as red spheres.
H-bond interactions are outlined by the dashed lines.
|
 |
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
F.Secundo,
S.Fialà,
M.W.Fraaije,
G.de Gonzalo,
M.Meli,
F.Zambianchi,
and
G.Ottolina
(2011).
Effects of water miscible organic solvents on the activity and conformation of the Baeyer-Villiger monooxygenases from Thermobifida fusca and Acinetobacter calcoaceticus: a comparative study.
|
| |
Biotechnol Bioeng,
108,
491-499.
|
 |
|
|
|
|
 |
H.J.Cho,
H.Y.Cho,
K.J.Kim,
M.H.Kim,
S.W.Kim,
and
B.S.Kang
(2011).
Structural and functional analysis of bacterial flavin-containing monooxygenase reveals its ping-pong-type reaction mechanism.
|
| |
J Struct Biol,
175,
39-48.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
B.N.Webb,
J.W.Ballinger,
E.Kim,
S.M.Belchik,
K.S.Lam,
B.Youn,
M.S.Nissen,
L.Xun,
and
C.Kang
(2010).
Characterization of chlorophenol 4-monooxygenase (TftD) and NADH:FAD oxidoreductase (TftC) of Burkholderia cepacia AC1100.
|
| |
J Biol Chem,
285,
2014-2027.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
D.J.Opperman,
and
M.T.Reetz
(2010).
Towards practical Baeyer-Villiger-monooxygenases: design of cyclohexanone monooxygenase mutants with enhanced oxidative stability.
|
| |
Chembiochem,
11,
2589-2596.
|
 |
|
|
|
|
 |
G.de Gonzalo,
M.D.Mihovilovic,
and
M.W.Fraaije
(2010).
Recent developments in the application of Baeyer-Villiger monooxygenases as biocatalysts.
|
| |
Chembiochem,
11,
2208-2231.
|
 |
|
|
|
|
 |
H.M.Dudek,
D.E.Torres Pazmiño,
C.Rodríguez,
G.de Gonzalo,
V.Gotor,
and
M.W.Fraaije
(2010).
Investigating the coenzyme specificity of phenylacetone monooxygenase from Thermobifida fusca.
|
| |
Appl Microbiol Biotechnol,
88,
1135-1143.
|
 |
|
|
|
|
 |
J.Rehdorf,
M.D.Mihovilovic,
M.W.Fraaije,
and
U.T.Bornscheuer
(2010).
Enzymatic synthesis of enantiomerically pure beta-amino ketones, beta-amino esters, and beta-amino alcohols with Baeyer-Villiger monooxygenases.
|
| |
Chemistry,
16,
9525-9535.
|
 |
|
|
|
|
 |
K.Geitner,
J.Rehdorf,
R.Snajdrova,
and
U.T.Bornscheuer
(2010).
Scale-up of Baeyer-Villiger monooxygenase-catalyzed synthesis of enantiopure compounds.
|
| |
Appl Microbiol Biotechnol,
88,
1087-1093.
|
 |
|
|
|
|
 |
S.Lutz
(2010).
Beyond directed evolution--semi-rational protein engineering and design.
|
| |
Curr Opin Biotechnol,
21,
734-743.
|
 |
|
|
|
|
 |
J.Jiang,
C.N.Tetzlaff,
S.Takamatsu,
M.Iwatsuki,
M.Komatsu,
H.Ikeda,
and
D.E.Cane
(2009).
Genome mining in Streptomyces avermitilis: A biochemical Baeyer-Villiger reaction and discovery of a new branch of the pentalenolactone family tree.
|
| |
Biochemistry,
48,
6431-6440.
|
 |
|
|
|
|
 |
J.Rehdorf,
C.L.Zimmer,
and
U.T.Bornscheuer
(2009).
Cloning, expression, characterization, and biocatalytic investigation of the 4-hydroxyacetophenone monooxygenase from Pseudomonas putida JD1.
|
| |
Appl Environ Microbiol,
75,
3106-3114.
|
 |
|
|
|
|
 |
J.T.Whitteck,
R.M.Cicchillo,
and
W.A.van der Donk
(2009).
Hydroperoxylation by hydroxyethylphosphonate dioxygenase.
|
| |
J Am Chem Soc,
131,
16225-16232.
|
 |
|
|
|
|
 |
M.P.Beam,
M.A.Bosserman,
N.Noinaj,
M.Wehenkel,
and
J.Rohr
(2009).
Crystal structure of Baeyer-Villiger monooxygenase MtmOIV, the key enzyme of the mithramycin biosynthetic pathway .
|
| |
Biochemistry,
48,
4476-4487.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.S.Motika,
J.Zhang,
X.Zheng,
K.Riedler,
and
J.R.Cashman
(2009).
Novel variants of the human flavin-containing monooxygenase 3 (FMO3) gene associated with trimethylaminuria.
|
| |
Mol Genet Metab,
97,
128-135.
|
 |
|
|
|
|
 |
S.K.Krueger,
M.C.Henderson,
L.K.Siddens,
J.E.VanDyke,
A.D.Benninghoff,
P.A.Karplus,
B.Furnes,
D.Schlenk,
and
D.E.Williams
(2009).
Characterization of sulfoxygenation and structural implications of human flavin-containing monooxygenase isoform 2 (FMO2.1) variants S195L and N413K.
|
| |
Drug Metab Dispos,
37,
1785-1791.
|
 |
|
|
|
|
 |
Y.C.Park,
C.E.Shaffer,
and
G.N.Bennett
(2009).
Microbial formation of esters.
|
| |
Appl Microbiol Biotechnol,
85,
13-25.
|
 |
|
|
|
|
 |
A.Alfieri,
E.Malito,
R.Orru,
M.W.Fraaije,
and
A.Mattevi
(2008).
Revealing the moonlighting role of NADP in the structure of a flavin-containing monooxygenase.
|
| |
Proc Natl Acad Sci U S A,
105,
6572-6577.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Kirschner,
and
U.T.Bornscheuer
(2008).
Directed evolution of a Baeyer-Villiger monooxygenase to enhance enantioselectivity.
|
| |
Appl Microbiol Biotechnol,
81,
465-472.
|
 |
|
|
|
|
 |
A.Taglieber,
F.Schulz,
F.Hollmann,
M.Rusek,
and
M.T.Reetz
(2008).
Light-driven biocatalytic oxidation and reduction reactions: scope and limitations.
|
| |
Chembiochem,
9,
565-572.
|
 |
|
|
|
|
 |
K.Fujino,
Y.Matsuda,
K.Ozawa,
T.Nishimura,
T.Koshiba,
M.W.Fraaije,
and
H.Sekiguchi
(2008).
NARROW LEAF 7 controls leaf shape mediated by auxin in rice.
|
| |
Mol Genet Genomics,
279,
499-507.
|
 |
|
|
|
|
 |
M.D.Mihovilovic,
B.Grötzl,
W.Kandioller,
A.Muskotál,
R.Snajdrova,
F.Rudroff,
and
H.Spreitzer
(2008).
Recombinant whole-cell mediated baeyer-villiger oxidation of perhydropyran-type ketones.
|
| |
Chem Biodivers,
5,
490-498.
|
 |
|
|
|
|
 |
M.D.Mihovilovic,
P.Kapitán,
and
P.Kapitánová
(2008).
Regiodivergent Baeyer-Villiger oxidation of fused ketones by recombinant whole-cell biocatalysts.
|
| |
ChemSusChem,
1,
143-148.
|
 |
|
|
|
|
 |
M.J.Moonen,
N.M.Kamerbeek,
A.H.Westphal,
S.A.Boeren,
D.B.Janssen,
M.W.Fraaije,
and
W.J.van Berkel
(2008).
Elucidation of the 4-hydroxyacetophenone catabolic pathway in Pseudomonas fluorescens ACB.
|
| |
J Bacteriol,
190,
5190-5198.
|
 |
|
|
|
|
 |
A.Alfieri,
F.Fersini,
N.Ruangchan,
M.Prongjit,
P.Chaiyen,
and
A.Mattevi
(2007).
Structure of the monooxygenase component of a two-component flavoprotein monooxygenase.
|
| |
Proc Natl Acad Sci U S A,
104,
1177-1182.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Kirschner,
J.Altenbuchner,
and
U.T.Bornscheuer
(2007).
Cloning, expression, and characterization of a Baeyer-Villiger monooxygenase from Pseudomonas fluorescens DSM 50106 in E. coli.
|
| |
Appl Microbiol Biotechnol,
73,
1065-1072.
|
 |
|
|
|
|
 |
J.Rehdorf,
A.Kirschner,
and
U.T.Bornscheuer
(2007).
Cloning, expression and characterization of a Baeyer-Villiger monooxygenase from Pseudomonas putida KT2440.
|
| |
Biotechnol Lett,
29,
1393-1398.
|
 |
|
|
|
|
 |
R.P.Hausinger
(2007).
New insights into acetone metabolism.
|
| |
J Bacteriol,
189,
671-673.
|
 |
|
|
|
|
 |
S.R.Kane,
A.Y.Chakicherla,
P.S.Chain,
R.Schmidt,
M.W.Shin,
T.C.Legler,
K.M.Scow,
F.W.Larimer,
S.M.Lucas,
P.M.Richardson,
and
K.R.Hristova
(2007).
Whole-genome analysis of the methyl tert-butyl ether-degrading beta-proteobacterium Methylibium petroleiphilum PM1.
|
| |
J Bacteriol,
189,
1931-1945.
|
 |
|
|
|
|
 |
T.Kotani,
H.Yurimoto,
N.Kato,
and
Y.Sakai
(2007).
Novel acetone metabolism in a propane-utilizing bacterium, Gordonia sp. strain TY-5.
|
| |
J Bacteriol,
189,
886-893.
|
 |
|
|
|
|
 |
V.Joosten,
and
W.J.van Berkel
(2007).
Flavoenzymes.
|
| |
Curr Opin Chem Biol,
11,
195-202.
|
 |
|
|
|
|
 |
D.Bonsor,
S.F.Butz,
J.Solomons,
S.Grant,
I.J.Fairlamb,
M.J.Fogg,
and
G.Grogan
(2006).
Ligation independent cloning (LIC) as a rapid route to families of recombinant biocatalysts from sequenced prokaryotic genomes.
|
| |
Org Biomol Chem,
4,
1252-1260.
|
 |
|
|
|
|
 |
H.Iwaki,
S.Wang,
S.Grosse,
H.Bergeron,
A.Nagahashi,
J.Lertvorachon,
J.Yang,
Y.Konishi,
Y.Hasegawa,
and
P.C.Lau
(2006).
Pseudomonad cyclopentadecanone monooxygenase displaying an uncommon spectrum of Baeyer-Villiger oxidations of cyclic ketones.
|
| |
Appl Environ Microbiol,
72,
2707-2720.
|
 |
|
|
|
|
 |
L.De Colibus,
and
A.Mattevi
(2006).
New frontiers in structural flavoenzymology.
|
| |
Curr Opin Struct Biol,
16,
722-728.
|
 |
|
|
|
|
 |
S.Eswaramoorthy,
J.B.Bonanno,
S.K.Burley,
and
S.Swaminathan
(2006).
Mechanism of action of a flavin-containing monooxygenase.
|
| |
Proc Natl Acad Sci U S A,
103,
9832-9837.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.Leungsakul,
G.R.Johnson,
and
T.K.Wood
(2006).
Protein engineering of the 4-methyl-5-nitrocatechol monooxygenase from Burkholderia sp. strain DNT for enhanced degradation of nitroaromatics.
|
| |
Appl Environ Microbiol,
72,
3933-3939.
|
 |
|
|
|
|
 |
C.Wang,
M.Gibson,
J.Rohr,
and
M.A.Oliveira
(2005).
Crystallization and X-ray diffraction properties of Baeyer-Villiger monooxygenase MtmOIV from the mithramycin biosynthetic pathway in Streptomyces argillaceus.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
61,
1023-1026.
|
 |
|
|
|
|
 |
F.Schulz,
F.Leca,
F.Hollmann,
and
M.T.Reetz
(2005).
Towards practical biocatalytic Baeyer-Villiger reactions: applying a thermostable enzyme in the gram-scale synthesis of optically-active lactones in a two-liquid-phase system.
|
| |
Beilstein J Org Chem,
1,
10.
|
 |
|
|
|
|
 |
G.de Gonzalo,
G.Ottolina,
G.Carrea,
and
M.W.Fraaije
(2005).
[Cp*Rh(bpy)(H2O)]2+ as a coenzyme substitute in enzymatic oxidations catalyzed by Baeyer-Villiger monooxygenases.
|
| |
Chem Commun (Camb),
(),
3724-3726.
|
 |
|
|
|
|
 |
S.K.Krueger,
and
D.E.Williams
(2005).
Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism.
|
| |
Pharmacol Ther,
106,
357-387.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

