 |
PDBsum entry 5mql
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
Crystal structure of dck mutant c3s in complex with masitinib and udp
|
|
Structure:
|
 |
Deoxycytidine kinase. Chain: a, b, c, d. Synonym: dck. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Gene: dck. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Resolution:
|
 |
|
3.25Å
|
R-factor:
|
0.219
|
R-free:
|
0.277
|
|
|
Authors:
|
 |
E.Rebuffet,K.Hammam,M.Saez-Ayala,L.Gros,S.Lopez,B.Hajem,M.Humbert, E.Baudelet,S.Audebert,S.Betzi,A.Lugari,S.Combes,D.Pez,S.Letard, C.Mansfield,A.Moussy,P.De Sepulveda,X.Morelli,P.Dubreuil
|
|
Key ref:
|
 |
K.Hammam
et al.
(2017).
Dual protein kinase and nucleoside kinase modulators for rationally designed polypharmacology.
Nat Commun,
8,
1420.
PubMed id:
|
 |
|
Date:
|
 |
|
20-Dec-16
|
Release date:
|
22-Nov-17
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P27707
(DCK_HUMAN) -
Deoxycytidine kinase from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
260 a.a.
231 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
 |
Secondary structure |
 |
|
*
PDB and UniProt seqs differ
at 2 residue positions (black
crosses)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.2.7.1.113
- deoxyguanosine kinase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
2'-deoxyguanosine + ATP = dGMP + ADP + H+
|
 |
 |
 |
 |
 |
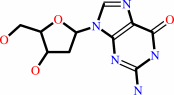 2'-deoxyguanosine
2'-deoxyguanosine
|
+
|
 ATP
ATP
|
=
|
dGMP
Bound ligand (Het Group name = )
matches with 79.31% similarity
|
+
|
ADP
Bound ligand (Het Group name = )
matches with 72.00% similarity
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.2.7.1.74
- deoxycytidine kinase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
2'-deoxycytidine + a ribonucleoside 5'-triphosphate = dCMP + a ribonucleoside 5'-diphosphate + H+
|
 |
 |
 |
 |
 |
 2'-deoxycytidine
2'-deoxycytidine
|
+
|
ribonucleoside 5'-triphosphate
|
=
|
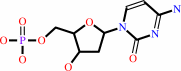 dCMP
dCMP
|
+
|
ribonucleoside 5'-diphosphate
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 4:
|
 |
E.C.2.7.1.76
- deoxyadenosine kinase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
2'-deoxyadenosine + ATP = dAMP + ADP + H+
|
 |
 |
 |
 |
 |
2'-deoxyadenosine
Bound ligand (Het Group name = )
matches with 65.22% similarity
|
+
|
 ATP
ATP
|
=
|
dAMP
Bound ligand (Het Group name = )
matches with 79.31% similarity
|
+
|
 ADP
ADP
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Nat Commun
8:1420
(2017)
|
|
PubMed id:
|
|
|
|

|
| |
|
Dual protein kinase and nucleoside kinase modulators for rationally designed polypharmacology.
|
|
K.Hammam,
M.Saez-Ayala,
E.Rebuffet,
L.Gros,
S.Lopez,
B.Hajem,
M.Humbert,
E.Baudelet,
S.Audebert,
S.Betzi,
A.Lugari,
S.Combes,
S.Letard,
N.Casteran,
C.Mansfield,
A.Moussy,
P.De Sepulveda,
X.Morelli,
P.Dubreuil.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Masitinib, a highly selective protein kinase inhibitor, can sensitise
gemcitabine-refractory cancer cell lines when used in combination with
gemcitabine. Here we report a reverse proteomic approach that identifies the
target responsible for this sensitisation: the deoxycytidine kinase (dCK).
Masitinib, as well as other protein kinase inhibitors, such as imatinib,
interact with dCK and provoke an unforeseen conformational-dependent activation
of this nucleoside kinase, modulating phosphorylation of nucleoside analogue
drugs. This phenomenon leads to an increase of prodrug phosphorylation of most
of the chemotherapeutic drugs activated by this nucleoside kinase. The
unforeseen dual activity of protein kinase inhibition/nucleoside kinase
activation could be of great therapeutic benefit, through either reducing
toxicity of therapeutic agents by maintaining effectiveness at lower doses or by
counteracting drug resistance initiated via down modulation of dCK target.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

