 |
PDBsum entry 5m8h
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
Atp phosphoribosyltransferase (hiszg atpprt) from psychrobacter arcticus
|
|
Structure:
|
 |
Atp phosphoribosyltransferase regulatory subunit. Chain: a, b, c, d. Engineered: yes. Other_details: some residues missing from model due to flexibility/poor electron density. Atp phosphoribosyltransferase. Chain: e, f, g, h. Synonym: atp-prtase. Engineered: yes.
|
|
Source:
|
 |
Psychrobacter arcticus (strain dsm 17307 / 273- 4). Organism_taxid: 259536. Gene: hisz, psyc_0676. Expressed in: escherichia coli bl21(de3). Expression_system_taxid: 469008. Gene: hisg, psyc_1903. Expression_system_variant: c43.
|
|
Resolution:
|
 |
|
2.34Å
|
R-factor:
|
0.230
|
R-free:
|
0.269
|
|
|
Authors:
|
 |
M.S.Alphey,Y.Ge,J.H.Naismith,R.G.Da Silva
|
|
Key ref:
|
 |
R.Stroek
et al.
(2017).
Kinetics and Structure of a Cold-Adapted Hetero-Octameric ATP Phosphoribosyltransferase.
Biochemistry,
56,
793-803.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
28-Oct-16
|
Release date:
|
06-Sep-17
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
Chains A, B, C, D:
E.C.?
|
|
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
Chains E, F, G, H:
E.C.2.4.2.17
- Atp phosphoribosyltransferase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
|
 |
 |
 |
 |
 |
Reaction:
|
 |
1-(5-phospho-beta-D-ribosyl)-ATP + diphosphate = 5-phospho-alpha-D-ribose 1-diphosphate + ATP
|
 |
 |
 |
 |
 |
1-(5-phospho-beta-D-ribosyl)-ATP
|
+
|
 diphosphate
diphosphate
|
=
|
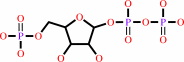 5-phospho-alpha-D-ribose 1-diphosphate
5-phospho-alpha-D-ribose 1-diphosphate
|
+
|
 ATP
ATP
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
56:793-803
(2017)
|
|
PubMed id:
|
|
|
|

|
| |
|
Kinetics and Structure of a Cold-Adapted Hetero-Octameric ATP Phosphoribosyltransferase.
|
|
R.Stroek,
Y.Ge,
P.D.Talbot,
M.K.Glok,
K.E.Bernaś,
C.M.Thomson,
E.R.Gould,
M.S.Alphey,
H.Liu,
G.J.Florence,
J.H.Naismith,
R.G.da Silva.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Adenosine 5'-triphosphate phosphoribosyltransferase (ATPPRT) catalyzes the first
step in histidine biosynthesis, the condensation of ATP and
5-phospho-α-d-ribosyl-1-pyrophosphate to generate
N1-(5-phospho-β-d-ribosyl)-ATP and inorganic pyrophosphate. The
enzyme is allosterically inhibited by histidine. Two forms of ATPPRT, encoded by
the hisG gene, exist in nature, depending on the species. The long form,
HisGL, is a single polypeptide chain with catalytic and regulatory
domains. The short form, HisGS, lacks a regulatory domain and cannot
bind histidine. HisGSinstead is found in complex with a regulatory
protein, HisZ, constituting the ATPPRT holoenzyme. HisZ triggers
HisGScatalytic activity while rendering it sensitive to allosteric
inhibition by histidine. Until recently, HisGSwas thought to be
catalytically inactive without HisZ. Here, recombinant HisGSand HisZ
from the psychrophilic bacterium Psychrobacter arcticus were independently
overexpressed and purified. The crystal structure of P. arcticus ATPPRT was
determined at 2.34 Å resolution, revealing an equimolar HisGS-HisZ
hetero-octamer. Steady-state kinetics indicate that both the ATPPRT holoenzyme
and HisGSare catalytically active. Surprisingly, HisZ confers only a
modest 2-4-fold increase in kcat. Reaction profiles for both enzymes
cannot be distinguished by31P nuclear magnetic resonance, indicating
that the same reaction is catalyzed. The temperature dependence of
kcatshows deviation from Arrhenius behavior at 308 K with the
holoenzyme. Interestingly, such deviation is detected only at 313 K with
HisGS. Thermal denaturation by CD spectroscopy resulted in
Tm's of 312 and 316 K for HisZ and HisGS, respectively,
suggesting that HisZ renders the ATPPRT complex more thermolabile. This is the
first characterization of a psychrophilic ATPPRT.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|

