 |
PDBsum entry 2dab

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
L201a mutant of d-amino acid aminotransferase complexed with pyridoxal-5'-phosphate
|
|
Structure:
|
 |
D-amino acid aminotransferase. Chain: a, b. Synonym: d-alanine aminotransferase, d-aspartate aminotransferase. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Bacillus sp.. Organism_taxid: 72579. Strain: ym-1. Expressed in: escherichia coli. Expression_system_taxid: 562.
|
|
Biol. unit:
|
 |
Homo-Dimer (from PDB file)
|
|
Resolution:
|
 |
|
2.00Å
|
R-factor:
|
0.218
|
R-free:
|
0.273
|
|
|
Authors:
|
 |
S.Sugio,A.Kashima,K.Kishimoto,D.Peisach,G.A.Petsko,D.Ringe, T.Yoshimura,N.Esaki
|
|
Key ref:
|
 |
S.Sugio
et al.
(1998).
Crystal structures of L201A mutant of D-amino acid aminotransferase at 2.0 A resolution: implication of the structural role of Leu201 in transamination.
Protein Eng,
11,
613-619.
PubMed id:
|
 |
|
Date:
|
 |
|
30-Nov-97
|
Release date:
|
03-Jun-98
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P19938
(DAAA_BACYM) -
D-alanine aminotransferase from Bacillus sp. (strain YM-1)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
283 a.a.
280 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.6.1.21
- D-amino-acid transaminase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
D-alanine + 2-oxoglutarate = D-glutamate + pyruvate
|
 |
 |
 |
 |
 |
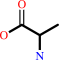 D-alanine
D-alanine
|
+
|
 2-oxoglutarate
2-oxoglutarate
|
=
|
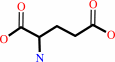 D-glutamate
D-glutamate
|
+
|
 pyruvate
pyruvate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
Pyridoxal 5'-phosphate
Bound ligand (Het Group name =
PLP)
matches with 93.75% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Protein Eng
11:613-619
(1998)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structures of L201A mutant of D-amino acid aminotransferase at 2.0 A resolution: implication of the structural role of Leu201 in transamination.
|
|
S.Sugio,
A.Kashima,
K.Kishimoto,
D.Peisach,
G.A.Petsko,
D.Ringe,
T.Yoshimura,
N.Esaki.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The leucine-to-alanine mutation at residue 201 of D-amino acid aminotransferase
provides a unique enzyme which gradually loses its activity while catalyzing the
normal transamination; the co-enzyme form is converted from pyridoxal
5'-phosphate to pyridoxamine 5'-phosphate upon the inactivation [Kishimoto,K.,
Yoshimura,T., Esaki,N., Sugio,S., Manning,J.M. and Soda,K. (1995) J. Biochem.,
117, 691-696]. Crystal structures of both co-enzyme forms of the mutant enzyme
have been determined at 2.0 A resolution: they are virtually identical, and are
quite similar to that of the wild-type enzyme. Significant differences in both
forms of the mutant are localized only on the bound co-enzyme, the side chains
of Lys145 and Tyr31, and a water molecule sitting on the putative substrate
binding site. Detailed comparisons of the structures of the mutant, together
with that of the pyridoxamine-5'-phosphate form of the wild-type enzyme, imply
that Leu201 would play a crucial role in the transamination reaction by keeping
the pyridoxyl ring in the proper location without disturbing its oscillating
motion, although the residue seems to not be especially important for the
structural integrity of the enzyme.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
H.Barreteau,
A.Kovac,
A.Boniface,
M.Sova,
S.Gobec,
and
D.Blanot
(2008).
Cytoplasmic steps of peptidoglycan biosynthesis.
|
| |
FEMS Microbiol Rev,
32,
168-207.
|
 |
|
|
|
|
 |
N.Yennawar,
J.Dunbar,
M.Conway,
S.Hutson,
and
G.Farber
(2001).
The structure of human mitochondrial branched-chain aminotransferase.
|
| |
Acta Crystallogr D Biol Crystallogr,
57,
506-515.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

