 |
PDBsum entry 1vkm

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Biosynthetic protein
|
PDB id
|
|

|

|
1vkm
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.4.2.1.70
- pseudouridylate synthase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
D-ribose 5-phosphate + uracil = psi-UMP + H2O
|
 |
 |
 |
 |
 |
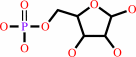 D-ribose 5-phosphate
D-ribose 5-phosphate
|
+
|
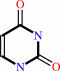 uracil
uracil
|
=
|
psi-UMP
|
+
|
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Proteins
59:864-868
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structure of an indigoidine synthase A (IndA)-like protein (TM1464) from Thermotoga maritima at 1.90 A resolution reveals a new fold.
|
|
I.Levin,
M.D.Miller,
R.Schwarzenbacher,
D.McMullan,
P.Abdubek,
E.Ambing,
T.Biorac,
J.Cambell,
J.M.Canaves,
H.J.Chiu,
A.M.Deacon,
M.DiDonato,
M.A.Elsliger,
A.Godzik,
C.Grittini,
S.K.Grzechnik,
J.Hale,
E.Hampton,
G.W.Han,
J.Haugen,
M.Hornsby,
L.Jaroszewski,
C.Karlak,
H.E.Klock,
E.Koesema,
A.Kreusch,
P.Kuhn,
S.A.Lesley,
A.Morse,
K.Moy,
E.Nigoghossian,
J.Ouyang,
R.Page,
K.Quijano,
R.Reyes,
A.Robb,
E.Sims,
G.Spraggon,
R.C.Stevens,
H.van den Bedem,
J.Velasquez,
J.Vincent,
X.Wang,
B.West,
G.Wolf,
Q.Xu,
O.Zagnitko,
K.O.Hodgson,
J.Wooley,
I.A.Wilson.
|
|
|

|
| |
ABSTRACT
|
|

|
| |
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1. Crystal structure of TM1464. (A) Stereo ribbon
diagram of T. maritima TM1464 color-coded from N-terminus (blue)
to C-terminus (red), showing the domain organization. Helices
H1-H12, and  -strands
( -strands
(  1- 1-
 11)
are indicated. (B) Diagram showing the secondary structure
elements in TM1464 superimposed on its primary sequence. The 11)
are indicated. (B) Diagram showing the secondary structure
elements in TM1464 superimposed on its primary sequence. The
 -helices,
3[10]-helices, -helices,
3[10]-helices,  -bulges,
and -bulges,
and  -turns
are indicated. The -turns
are indicated. The  -sheet
strands are indicated by a red A, and -sheet
strands are indicated by a red A, and  -hairpins
are depicted as red loops. -hairpins
are depicted as red loops.
|
 |
Figure 2.
Figure 2. (A) The TM1464 trimer viewed along the 3-fold axis.
One subunit is shown in blue. The 3 UNLs bound to the putative
active sites are shown as red spheres. (B) TM1464 shown in
surface representation illustrating the narrow active site
pocket, with the bound UNL in ball-and-stick configuration. (C)
Close-up of the putative active site. TM1464 is shown in ribbon
representation with the UNL, manganese, and interacting residues
and waters in ball-and-stick configuration (see text). A
SigmaA-weighted Fo-Fc omit map contoured at 3.0  is
shown for the UNL, manganese, and coordinating waters. Putative
H-bond interactions between the UNL and the protein are shown in
yellow dotted lines. is
shown for the UNL, manganese, and coordinating waters. Putative
H-bond interactions between the UNL and the protein are shown in
yellow dotted lines.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from John Wiley & Sons, Inc.:
Proteins
(2005,
59,
864-868)
copyright 2005.
|
|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
E.S.Julfayev,
R.J.McLaughlin,
Y.P.Tao,
and
W.A.McLaughlin
(2011).
A new approach to assess and predict the functional roles of proteins across all known structures.
|
| |
J Struct Funct Genomics,
12,
9.
|
 |
|
|
|
|
 |
A.Preumont,
K.Snoussi,
V.Stroobant,
J.F.Collet,
and
E.Van Schaftingen
(2008).
Molecular identification of pseudouridine-metabolizing enzymes.
|
| |
J Biol Chem,
283,
25238-25246.
|
 |
|
|
|
|
 |
T.Oja,
K.Palmu,
H.Lehmussola,
O.Leppäranta,
K.Hännikäinen,
J.Niemi,
P.Mäntsälä,
and
M.Metsä-Ketelä
(2008).
Characterization of the alnumycin gene cluster reveals unusual gene products for pyran ring formation and dioxan biosynthesis.
|
| |
Chem Biol,
15,
1046-1057.
|
 |
|
|
|
|
 |
H.Takahashi,
T.Kumagai,
K.Kitani,
M.Mori,
Y.Matoba,
and
M.Sugiyama
(2007).
Cloning and characterization of a Streptomyces single module type non-ribosomal peptide synthetase catalyzing a blue pigment synthesis.
|
| |
J Biol Chem,
282,
9073-9081.
|
 |
|
|
|
|
 |
S.B.Conners,
E.F.Mongodin,
M.R.Johnson,
C.I.Montero,
K.E.Nelson,
and
R.M.Kelly
(2006).
Microbial biochemistry, physiology, and biotechnology of hyperthermophilic Thermotoga species.
|
| |
FEMS Microbiol Rev,
30,
872-905.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

