 |
PDBsum entry 1fs8

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1fs8
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
CytochromE C nitrite reductase from wolinella succinogenes-sulfate complex
|
|
Structure:
|
 |
CytochromE C nitrite reductase. Chain: a
|
|
Source:
|
 |
Wolinella succinogenes. Organism_taxid: 844
|
|
Biol. unit:
|
 |
 Dimer (from PDB file)
Dimer (from PDB file)
|
|
Resolution:
|
 |
|
1.60Å
|
R-factor:
|
0.184
|
R-free:
|
0.206
|
|
|
Authors:
|
 |
O.Einsle,P.Stach,A.Messerschmidt,J.Simon,A.Kroeger,R.Huber, P.M.H.Kroneck
|
Key ref:

|
 |
O.Einsle
et al.
(2000).
Cytochrome c nitrite reductase from Wolinella succinogenes. Structure at 1.6 A resolution, inhibitor binding, and heme-packing motifs.
J Biol Chem,
275,
39608-39616.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
08-Sep-00
|
Release date:
|
17-Jan-01
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q9S1E5
(NRFA_WOLSU) -
Cytochrome c-552 from Wolinella succinogenes (strain ATCC 29543 / DSM 1740 / CCUG 13145 / JCM 31913 / LMG 7466 / NCTC 11488 / FDC 602W)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
507 a.a.
471 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.7.2.2
- nitrite reductase (cytochrome; ammonia-forming).
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
6 Fe(III)-[cytochrome c] + NH4+ + 2 H2O = 6 Fe(II)-[cytochrome c] + nitrite + 8 H+
|
 |
 |
 |
 |
 |
6
×
Fe(III)-[cytochrome c]
|
+
|
NH4(+)
|
+
|
2
×
H2O
|
=
|
6
×
Fe(II)-[cytochrome c]
|
+
|
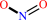 nitrite
nitrite
|
+
|
8
×
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Ca(2+); Heme
|
 |
 |
 |
 |
 |
Ca(2+)
|
Heme
Bound ligand (Het Group name =
HEC)
matches with 95.45% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
275:39608-39616
(2000)
|
|
PubMed id:
|
|
|
|

|
| |
|
Cytochrome c nitrite reductase from Wolinella succinogenes. Structure at 1.6 A resolution, inhibitor binding, and heme-packing motifs.
|
|
O.Einsle,
P.Stach,
A.Messerschmidt,
J.Simon,
A.Kröger,
R.Huber,
P.M.Kroneck.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Cytochrome c nitrite reductase catalyzes the 6-electron reduction of nitrite to
ammonia. This second part of the respiratory pathway of nitrate ammonification
is a key step in the biological nitrogen cycle. The x-ray structure of the
enzyme from the epsilon-proteobacterium Wolinella succinogenes has been solved
to a resolution of 1.6 A. It is a pentaheme c-type cytochrome whose heme groups
are packed in characteristic motifs that also occur in other multiheme
cytochromes. Structures of W. succinogenes nitrite reductase have been obtained
with water bound to the active site heme iron as well as complexes with two
inhibitors, sulfate and azide, whose binding modes and inhibitory functions
differ significantly. Cytochrome c nitrite reductase is part of a highly
optimized respiratory system found in a wide range of Gram-negative bacteria. It
reduces both anionic and neutral substrates at the distal side of a
lysine-coordinated high-spin heme group, which is accessible through two
different channels, allowing for a guided flow of reaction educt and product.
Based on sequence comparison and secondary structure prediction, we have
demonstrated that cytochrome c nitrite reductases constitute a protein family of
high structural similarity.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Fig. 1. Overall structure of nitrite reductase. A, front
view of the dimer of W. succinogenes cytochrome c nitrite
reductase. One monomer forms the asymmetric unit of the
crystals. Dimer formation is mediated by the central helical
segments. B, the arrangement of heme groups in the same
orientation as in A. In the left monomer, hemes are numbered
according to their attachment to the protein chain. In the right
monomer, distances are given between the iron atoms. Images were
produced with Molscript (32) and Raster3D (33).
|
 |
Figure 5.
Fig. 5. Heme-packing motifs. Superposition of the heme
groups of nitrite reductase (gray) and hydroxylamine
oxidoreductase (black) numbered according to their attachment to
the protein chain. With the exception of the active site heme (1
in NiR, 4 in hydroxylamine oxidoreductase), all heme groups form
di-heme elbow motifs ( circles), which are connected via a
parallel stacking arrangement similar to the one observed in
split-Soret cytochrome c (ovals). Images were produced using
Molscript (32).
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2000,
275,
39608-39616)
copyright 2000.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
C.Lockwood,
J.N.Butt,
T.A.Clarke,
and
D.J.Richardson
(2011).
Molecular interactions between multihaem cytochromes: probing the protein-protein interactions between pentahaem cytochromes of a nitrite reductase complex.
|
| |
Biochem Soc Trans,
39,
263-268.
|
 |
|
|
|
|
 |
D.Bykov,
and
F.Neese
(2011).
Substrate binding and activation in the active site of cytochrome c nitrite reductase: a density functional study.
|
| |
J Biol Inorg Chem,
16,
417-430.
|
 |
|
|
|
|
 |
A.A.Trofimov,
K.M.Polyakov,
K.M.Boyko,
T.V.Tikhonova,
T.N.Safonova,
A.V.Tikhonov,
A.N.Popov,
and
V.O.Popov
(2010).
Structures of complexes of octahaem cytochrome c nitrite reductase from Thioalkalivibrio nitratireducens with sulfite and cyanide.
|
| |
Acta Crystallogr D Biol Crystallogr,
66,
1043-1047.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
G.L.Kemp,
T.A.Clarke,
S.J.Marritt,
C.Lockwood,
S.R.Poock,
A.M.Hemmings,
D.J.Richardson,
M.R.Cheesman,
and
J.N.Butt
(2010).
Kinetic and thermodynamic resolution of the interactions between sulfite and the pentahaem cytochrome NrfA from Escherichia coli.
|
| |
Biochem J,
431,
73-80.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
L.J.Smith,
A.Kahraman,
and
J.M.Thornton
(2010).
Heme proteins--diversity in structural characteristics, function, and folding.
|
| |
Proteins,
78,
2349-2368.
|
 |
|
|
|
|
 |
M.Kern,
F.Eisel,
J.Scheithauer,
R.G.Kranz,
and
J.Simon
(2010).
Substrate specificity of three cytochrome c haem lyase isoenzymes from Wolinella succinogenes: unconventional haem c binding motifs are not sufficient for haem c attachment by NrfI and CcsA1.
|
| |
Mol Microbiol,
75,
122-137.
|
 |
|
|
|
|
 |
S.Sharma,
G.Cavallaro,
and
A.Rosato
(2010).
A systematic investigation of multiheme c-type cytochromes in prokaryotes.
|
| |
J Biol Inorg Chem,
15,
559-571.
|
 |
|
|
|
|
 |
C.Fufezan,
J.Zhang,
and
M.R.Gunner
(2008).
Ligand preference and orientation in b- and c-type heme-binding proteins.
|
| |
Proteins,
73,
690-704.
|
 |
|
|
|
|
 |
D.Han,
K.Kim,
J.Oh,
J.Park,
and
Y.Kim
(2008).
TPR domain of NrfG mediates complex formation between heme lyase and formate-dependent nitrite reductase in Escherichia coli O157:H7.
|
| |
Proteins,
70,
900-914.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
D.Heitmann,
and
O.Einsle
(2008).
Pseudo-merohedral twinning in crystals of the dihaem c-type cytochrome DHC2 from Geobacter sulfurreducens.
|
| |
Acta Crystallogr D Biol Crystallogr,
64,
993-999.
|
 |
|
|
|
|
 |
H.J.Kim,
A.Zatsman,
A.K.Upadhyay,
M.Whittaker,
D.Bergmann,
M.P.Hendrich,
and
A.B.Hooper
(2008).
Membrane tetraheme cytochrome c(m552) of the ammonia-oxidizing nitrosomonas europaea: a ubiquinone reductase.
|
| |
Biochemistry,
47,
6539-6551.
|
 |
|
|
|
|
 |
J.S.Zhao,
D.Manno,
and
J.Hawari
(2008).
Regulation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) metabolism in Shewanella halifaxensis HAW-EB4 by terminal electron acceptor and involvement of c-type cytochrome.
|
| |
Microbiology,
154,
1026-1037.
|
 |
|
|
|
|
 |
T.V.Tikhonova,
E.S.Slutskaya,
A.A.Filimonenkov,
K.M.Boyko,
S.Y.Kleimenov,
P.V.Konarev,
K.M.Polyakov,
D.I.Svergun,
A.A.Trofimov,
V.G.Khomenkov,
R.A.Zvyagilskaya,
and
V.O.Popov
(2008).
Isolation and oligomeric composition of cytochrome c nitrite reductase from the haloalkaliphilic bacterium Thioalkalivibrio nitratireducens.
|
| |
Biochemistry (Mosc),
73,
164-170.
|
 |
|
|
|
|
 |
M.S.Pittman,
K.T.Elvers,
L.Lee,
M.A.Jones,
R.K.Poole,
S.F.Park,
and
D.J.Kelly
(2007).
Growth of Campylobacter jejuni on nitrate and nitrite: electron transport to NapA and NrfA via NrfH and distinct roles for NrfA and the globin Cgb in protection against nitrosative stress.
|
| |
Mol Microbiol,
63,
575-590.
|
 |
|
|
|
|
 |
M.L.Rodrigues,
T.F.Oliveira,
I.A.Pereira,
and
M.Archer
(2006).
X-ray structure of the membrane-bound cytochrome c quinol dehydrogenase NrfH reveals novel haem coordination.
|
| |
EMBO J,
25,
5951-5960.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.L.Rodrigues,
T.Oliveira,
P.M.Matias,
I.C.Martins,
F.M.Valente,
I.A.Pereira,
and
M.Archer
(2006).
Crystallization and preliminary structure determination of the membrane-bound complex cytochrome c nitrite reductase from Desulfovibrio vulgaris Hildenborough.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
62,
565-568.
|
 |
|
|
|
|
 |
T.Teschner,
L.Yatsunyk,
V.Schünemann,
H.Paulsen,
H.Winkler,
C.Hu,
W.R.Scheidt,
F.A.Walker,
and
A.X.Trautwein
(2006).
Models of the membrane-bound cytochromes: mössbauer spectra of crystalline low-spin ferriheme complexes having axial ligand plane dihedral angles ranging from 0 degree to 90 degrees.
|
| |
J Am Chem Soc,
128,
1379-1389.
|
 |
|
|
|
|
 |
C.G.Mowat,
and
S.K.Chapman
(2005).
Multi-heme cytochromes--new structures, new chemistry.
|
| |
Dalton Trans,
(),
3381-3389.
|
 |
|
|
|
|
 |
D.J.Bergmann,
A.B.Hooper,
and
M.G.Klotz
(2005).
Structure and sequence conservation of hao cluster genes of autotrophic ammonia-oxidizing bacteria: evidence for their evolutionary history.
|
| |
Appl Environ Microbiol,
71,
5371-5382.
|
 |
|
|
|
|
 |
S.Kura,
S.Kuwata,
and
T.Ikariya
(2005).
N-Methylhydroxylamido(1-)- and nitrosomethaneruthenium complexes derived from nitrosyl complexes: reversible N-protonation of an N-coordinated nitrosoalkane.
|
| |
Angew Chem Int Ed Engl,
44,
6406-6409.
|
 |
|
|
|
|
 |
C.G.Mowat,
E.Rothery,
C.S.Miles,
L.McIver,
M.K.Doherty,
K.Drewette,
P.Taylor,
M.D.Walkinshaw,
S.K.Chapman,
and
G.A.Reid
(2004).
Octaheme tetrathionate reductase is a respiratory enzyme with novel heme ligation.
|
| |
Nat Struct Mol Biol,
11,
1023-1024.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
P.R.Pokkuluri,
Y.Y.Londer,
N.E.Duke,
J.Erickson,
M.Pessanha,
C.A.Salgueiro,
and
M.Schiffer
(2004).
Structure of a novel c7-type three-heme cytochrome domain from a multidomain cytochrome c polymer.
|
| |
Protein Sci,
13,
1684-1692.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.Simon,
M.Sänger,
S.C.Schuster,
and
R.Gross
(2003).
Electron transport to periplasmic nitrate reductase (NapA) of Wolinella succinogenes is independent of a NapC protein.
|
| |
Mol Microbiol,
49,
69-79.
|
 |
|
|
|
|
 |
M.G.Almeida,
S.Macieira,
L.L.Gonçalves,
R.Huber,
C.A.Cunha,
M.J.Romão,
C.Costa,
J.Lampreia,
J.J.Moura,
and
I.Moura
(2003).
The isolation and characterization of cytochrome c nitrite reductase subunits (NrfA and NrfH) from Desulfovibrio desulfuricans ATCC 27774. Re-evaluation of the spectroscopic data and redox properties.
|
| |
Eur J Biochem,
270,
3904-3915.
|
 |
|
|
|
|
 |
J.Simon
(2002).
Enzymology and bioenergetics of respiratory nitrite ammonification.
|
| |
FEMS Microbiol Rev,
26,
285-309.
|
 |
|
|
|
|
 |
M.J.Sellars,
S.J.Hall,
and
D.J.Kelly
(2002).
Growth of Campylobacter jejuni supported by respiration of fumarate, nitrate, nitrite, trimethylamine-N-oxide, or dimethyl sulfoxide requires oxygen.
|
| |
J Bacteriol,
184,
4187-4196.
|
 |
|
|
|
|
 |
O.Einsle,
P.Stach,
A.Messerschmidt,
O.Klimmek,
J.Simon,
A.Kröger,
and
P.M.Kroneck
(2002).
Crystallization and preliminary X-ray analysis of the membrane-bound cytochrome c nitrite reductase complex (NrfHA) from Wolinella succinogenes.
|
| |
Acta Crystallogr D Biol Crystallogr,
58,
341-342.
|
 |
|
|
|
|
 |
R.Pisa,
T.Stein,
R.Eichler,
R.Gross,
and
J.Simon
(2002).
The nrfI gene is essential for the attachment of the active site haem group of Wolinella succinogenes cytochrome c nitrite reductase.
|
| |
Mol Microbiol,
43,
763-770.
|
 |
|
|
|
|
 |
V.A.Bamford,
H.C.Angove,
H.E.Seward,
A.J.Thomson,
J.A.Cole,
J.N.Butt,
A.M.Hemmings,
and
D.J.Richardson
(2002).
Structure and spectroscopy of the periplasmic cytochrome c nitrite reductase from Escherichia coli.
|
| |
Biochemistry,
41,
2921-2931.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
I.Moura,
and
J.J.Moura
(2001).
Structural aspects of denitrifying enzymes.
|
| |
Curr Opin Chem Biol,
5,
168-175.
|
 |
|
|
|
|
 |
J.Simon,
R.Pisa,
T.Stein,
R.Eichler,
O.Klimmek,
and
R.Gross
(2001).
The tetraheme cytochrome c NrfH is required to anchor the cytochrome c nitrite reductase (NrfA) in the membrane of Wolinella succinogenes.
|
| |
Eur J Biochem,
268,
5776-5782.
|
 |
|
|
|
|
 |
O.Einsle,
S.Foerster,
K.Mann,
G.Fritz,
A.Messerschmidt,
and
P.M.Kroneck
(2001).
Spectroscopic investigation and determination of reactivity and structure of the tetraheme cytochrome c3 from Desulfovibrio desulfuricans Essex 6.
|
| |
Eur J Biochem,
268,
3028-3035.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

