
|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B:
E.C.4.2.1.20
- tryptophan synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Tryptophan Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
(1S,2R)-1-C-(indol-3-yl)glycerol 3-phosphate + L-serine = D-glyceraldehyde 3-phosphate + L-tryptophan + H2O
|
 |
 |
 |
 |
 |
(1S,2R)-1-C-(indol-3-yl)glycerol 3-phosphate
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
 L-serine
L-serine
|
=
|
 D-glyceraldehyde 3-phosphate
D-glyceraldehyde 3-phosphate
|
+
|
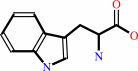 L-tryptophan
L-tryptophan
|
+
|
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
Pyridoxal 5'-phosphate
Bound ligand (Het Group name =
PLP)
matches with 93.75% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
38:16469-16480
(1999)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structure of wild-type tryptophan synthase complexed with the natural substrate indole-3-glycerol phosphate.
|
|
M.Weyand,
I.Schlichting.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
We used freeze trapping to stabilize the Michaelis complex of wild-type
tryptophan synthase and the alpha-subunit substrate indole-3-glycerol phosphate
(IGP) and determined its structure to 1. 8 A resolution. In addition, we
determined the 1.4 A resolution structure of the complex with indole-3-propanole
phosphate (IPP), a noncleavable IGP analogue. The interaction of the 3'-hydroxyl
of IGP with the catalytic alphaGlu49 leads to a twisting of the propane chain
and to a repositioning of the indole ring compared to IPP. Concomitantly, the
catalytic alphaAsp60 rotates resulting in a translocation of the COMM domain
[betaGly102-betaGly189, for definition see Schneider et al. (1998) Biochemistry
37, 5394-5406] in a direction opposite to the one in the IPP complex. This
results in loss of the allosteric sodium ion bound at the beta-subunit and an
opening of the beta-active site, thereby making the cofactor pyridoxal
5'-phosphate (PLP) accessible to solvent and thus serine binding. These findings
form the structural basis for the information transfer from the alpha- to the
beta-subunit and may explain the affinity increase of the beta-active site for
serine upon IGP binding.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
K.Nishio,
K.Ogasahara,
Y.Morimoto,
T.Tsukihara,
S.J.Lee,
and
K.Yutani
(2010).
Large conformational changes in the Escherichia coli tryptophan synthase beta(2) subunit upon pyridoxal 5'-phosphate binding.
|
| |
FEBS J,
277,
2157-2170.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Q.Fatmi,
and
C.E.Chang
(2010).
The role of oligomerization and cooperative regulation in protein function: the case of tryptophan synthase.
|
| |
PLoS Comput Biol,
6,
e1000994.
|
 |
|
|
|
|
 |
T.R.Barends,
M.F.Dunn,
and
I.Schlichting
(2008).
Tryptophan synthase, an allosteric molecular factory.
|
| |
Curr Opin Chem Biol,
12,
593-600.
|
 |
|
|
|
|
 |
G.McClarty,
H.D.Caldwell,
and
D.E.Nelson
(2007).
Chlamydial interferon gamma immune evasion influences infection tropism.
|
| |
Curr Opin Microbiol,
10,
47-51.
|
 |
|
|
|
|
 |
J.T.Huang,
J.P.Cheng,
and
H.Chen
(2007).
Secondary structure length as a determinant of folding rate of proteins with two- and three-state kinetics.
|
| |
Proteins,
67,
12-17.
|
 |
|
|
|
|
 |
R.Merkl
(2007).
Modelling the evolution of the archeal tryptophan synthase.
|
| |
BMC Evol Biol,
7,
59.
|
 |
|
|
|
|
 |
E.Di Cera
(2006).
A structural perspective on enzymes activated by monovalent cations.
|
| |
J Biol Chem,
281,
1305-1308.
|
 |
|
|
|
|
 |
H.Wood,
C.Roshick,
and
G.McClarty
(2004).
Tryptophan recycling is responsible for the interferon-gamma resistance of Chlamydia psittaci GPIC in indoleamine dioxygenase-expressing host cells.
|
| |
Mol Microbiol,
52,
903-916.
|
 |
|
|
|
|
 |
P.Storici,
D.De Biase,
F.Bossa,
S.Bruno,
A.Mozzarelli,
C.Peneff,
R.B.Silverman,
and
T.Schirmer
(2004).
Structures of gamma-aminobutyric acid (GABA) aminotransferase, a pyridoxal 5'-phosphate, and [2Fe-2S] cluster-containing enzyme, complexed with gamma-ethynyl-GABA and with the antiepilepsy drug vigabatrin.
|
| |
J Biol Chem,
279,
363-373.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
Y.Hioki,
K.Ogasahara,
S.J.Lee,
J.Ma,
M.Ishida,
Y.Yamagata,
Y.Matsuura,
M.Ota,
M.Ikeguchi,
S.Kuramitsu,
and
K.Yutani
(2004).
The crystal structure of the tryptophan synthase beta subunit from the hyperthermophile Pyrococcus furiosus. Investigation of stabilization factors.
|
| |
Eur J Biochem,
271,
2624-2635.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Osborne,
Q.Teng,
E.W.Miles,
and
R.S.Phillips
(2003).
Detection of open and closed conformations of tryptophan synthase by 15N-heteronuclear single-quantum coherence nuclear magnetic resonance of bound 1-15N-L-tryptophan.
|
| |
J Biol Chem,
278,
44083-44090.
|
 |
|
|
|
|
 |
H.D.Caldwell,
H.Wood,
D.Crane,
R.Bailey,
R.B.Jones,
D.Mabey,
I.Maclean,
Z.Mohammed,
R.Peeling,
C.Roshick,
J.Schachter,
A.W.Solomon,
W.E.Stamm,
R.J.Suchland,
L.Taylor,
S.K.West,
T.C.Quinn,
R.J.Belland,
and
G.McClarty
(2003).
Polymorphisms in Chlamydia trachomatis tryptophan synthase genes differentiate between genital and ocular isolates.
|
| |
J Clin Invest,
111,
1757-1769.
|
 |
|
|
|
|
 |
K.Ogasahara,
M.Ishida,
and
K.Yutani
(2003).
Stimulated interaction between and subunits of tryptophan synthase from hyperthermophile enhances its thermal stability.
|
| |
J Biol Chem,
278,
8922-8928.
|
 |
|
|
|
|
 |
C.Fehlner-Gardiner,
C.Roshick,
J.H.Carlson,
S.Hughes,
R.J.Belland,
H.D.Caldwell,
and
G.McClarty
(2002).
Molecular basis defining human Chlamydia trachomatis tissue tropism. A possible role for tryptophan synthase.
|
| |
J Biol Chem,
277,
26893-26903.
|
 |
|
|
|
|
 |
G.Xie,
C.Forst,
C.Bonner,
and
R.A.Jensen
(2002).
Significance of two distinct types of tryptophan synthase beta chain in Bacteria, Archaea and higher plants.
|
| |
Genome Biol,
3,
RESEARCH0004.
|
 |
|
|
|
|
 |
M.Weyand,
I.Schlichting,
A.Marabotti,
and
A.Mozzarelli
(2002).
Crystal structures of a new class of allosteric effectors complexed to tryptophan synthase.
|
| |
J Biol Chem,
277,
10647-10652.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Weyand,
I.Schlichting,
P.Herde,
A.Marabotti,
and
A.Mozzarelli
(2002).
Crystal structure of the beta Ser178--> Pro mutant of tryptophan synthase. A "knock-out" allosteric enzyme.
|
| |
J Biol Chem,
277,
10653-10660.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Hettwer,
and
R.Sterner
(2002).
A novel tryptophan synthase beta-subunit from the hyperthermophile Thermotoga maritima. Quaternary structure, steady-state kinetics, and putative physiological role.
|
| |
J Biol Chem,
277,
8194-8201.
|
 |
|
|
|
|
 |
E.W.Miles
(2001).
Tryptophan synthase: a multienzyme complex with an intramolecular tunnel.
|
| |
Chem Rec,
1,
140-151.
|
 |
|
|
|
|
 |
M.Frey,
C.Stettner,
P.W.Pare,
E.A.Schmelz,
J.H.Tumlinson,
and
A.Gierl
(2000).
An herbivore elicitor activates the gene for indole emission in maize.
|
| |
Proc Natl Acad Sci U S A,
97,
14801-14806.
|
 |
|
|
|
|
 |
S.S.Parikh,
G.Walcher,
G.D.Jones,
G.Slupphaug,
H.E.Krokan,
G.M.Blackburn,
and
J.A.Tainer
(2000).
Uracil-DNA glycosylase-DNA substrate and product structures: conformational strain promotes catalytic efficiency by coupled stereoelectronic effects.
|
| |
Proc Natl Acad Sci U S A,
97,
5083-5088.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

