 |
PDBsum entry 1q4g

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1q4g
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
2.0 angstrom crystal structure of ovine prostaglandin h2 synthase-1, in complex with alpha-methyl-4-biphenylacetic acid
|
|
Structure:
|
 |
Prostaglandin g/h synthase 1. Chain: a, b. Synonym: cyclooxygenase -1, cox-1, prostaglandin-endoperoxide synthase 1, prostaglandin h2 synthase 1, pgh synthase 1, pghs-1, phs 1. Ec: 1.14.99.1
|
|
Source:
|
 |
Ovis aries. Sheep. Organism_taxid: 9940. Organ: seminal vesicle
|
|
Biol. unit:
|
 |
Dimer (from
)
|
|
Resolution:
|
 |
|
2.00Å
|
R-factor:
|
0.217
|
R-free:
|
0.231
|
|
|
Authors:
|
 |
K.Gupta,B.S.Selinksy,C.J.Kaub,A.K.Katz,P.J.Loll
|
Key ref:

|
 |
K.Gupta
et al.
(2004).
The 2.0 A resolution crystal structure of prostaglandin H2 synthase-1: structural insights into an unusual peroxidase.
J Mol Biol,
335,
503-518.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
03-Aug-03
|
Release date:
|
06-Jan-04
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P05979
(PGH1_SHEEP) -
Prostaglandin G/H synthase 1 from Ovis aries
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
600 a.a.
553 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 2 residue positions (black
crosses)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.14.99.1
- prostaglandin-endoperoxide synthase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
(5Z,8Z,11Z,14Z)-eicosatetraenoate + AH2 + 2 O2 = prostaglandin H2 + A + H2O
|
 |
 |
 |
 |
 |
(5Z,8Z,11Z,14Z)-eicosatetraenoate
|
+
|
AH2
|
+
|
 2
×
O2
2
×
O2
|
=
|
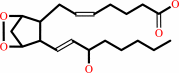 prostaglandin H2
prostaglandin H2
|
+
|
|
+
|
H2O
Bound ligand (Het Group name = )
matches with 51.11% similarity
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Mol Biol
335:503-518
(2004)
|
|
PubMed id:
|
|
|
|

|
| |
|
The 2.0 A resolution crystal structure of prostaglandin H2 synthase-1: structural insights into an unusual peroxidase.
|
|
K.Gupta,
B.S.Selinsky,
C.J.Kaub,
A.K.Katz,
P.J.Loll.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Prostaglandin H2 synthase (EC 1.14.99.1) is an integral membrane enzyme
containing a cyclooxygenase site, which is the target for the non-steroidal
anti-inflammatory drugs, and a spatially distinct peroxidase site. Previous
crystallographic studies of this clinically important drug target have been
hindered by low resolution. We present here the 2.0 A resolution X-ray crystal
structure of ovine prostaglandin H2 synthase-1 in complex with
alpha-methyl-4-biphenylacetic acid, a defluorinated analog of the non-steroidal
anti-inflammatory drug flurbiprofen. Detergent molecules are seen to bind to the
protein's membrane-binding domain, and their positions suggest the depth to
which this domain is likely to penetrate into the lipid bilayer. The relation of
the enzyme's proximal heme ligand His388 to the heme iron is atypical for a
peroxidase; the iron-histidine bond is unusually long and a substantial tilt
angle is observed between the heme and imidazole planes. A molecule of glycerol,
used as a cryoprotectant during diffraction experiments, is seen to bind in the
peroxidase site, offering the first view of any ligand in this active site.
Insights gained from glycerol binding may prove useful in the design of a
peroxidase-specific ligand.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Figure 3. Stereoscopic diagram of the PGHS monomer. The
structural domains are colored as follows: the EGF-like domain,
green; the membrane-binding domain, yellow; and the catalytic
domain, blue. The cyclooxygenase inhibitor
a-methyl-4-biphenylacetic acid is depicted as an orange
space-filling model, and glycerol molecules are depicted as pink
space-filling models. PGHS features three sites of
glycosylation, Asn68, Asn144, and Asn410. The carbohydrate
molecules are shown in yellow ball-and-stick representations.
Several molecules of the non-ionic detergent b-OG (black and red
ball-and-stick) used in the crystallization of PGHS are bound
near the membrane-binding domain. This Figure was prepared using
MOLSCRIPT.[85.]
|
 |
Figure 9.
Figure 9. (a) Stereoscopic view of the peroxidase active
site of PGHS-1. Glycerol is rendered as a ball-and-stick model.
Small individual spheres represent water molecules, while the
large sphere at the center of the porphyrin represents the iron
atom. This Figure was prepared using MOLSCRIPT.[85.] (b)
Simulated annealing omit map density for glycerol bound within
the peroxidase active site of PGHS (F[o] -F[c] map contoured at
4.0 s). The view is from above the peroxidase active site,
looking down onto the heme. The porphyrin can be seen in the
background of the Figure. This panel was prepared using PYMOL
(http://www.pymol.org).
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Elsevier:
J Mol Biol
(2004,
335,
503-518)
copyright 2004.
|
|
| |
Figures were
selected
by the author.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.L.Tsai,
and
R.J.Kulmacz
(2010).
Prostaglandin H synthase: resolved and unresolved mechanistic issues.
|
| |
Arch Biochem Biophys,
493,
103-124.
|
 |
|
|
|
|
 |
D.Raffa,
O.Migliara,
B.Maggio,
F.Plescia,
S.Cascioferro,
M.G.Cusimano,
G.Tringali,
C.Cannizzaro,
and
F.Plescia
(2010).
Pyrazolobenzotriazinone derivatives as COX inhibitors: synthesis, biological activity, and molecular-modeling studies.
|
| |
Arch Pharm (Weinheim),
343,
631-638.
|
 |
|
|
|
|
 |
S.Alcaro,
A.Artese,
M.Botta,
A.T.Zizzari,
F.Orallo,
F.Ortuso,
S.Schenone,
C.Brullo,
and
M.Yáñez
(2010).
Hit identification and biological evaluation of anticancer pyrazolopyrimidines endowed with anti-inflammatory activity.
|
| |
ChemMedChem,
5,
1242-1246.
|
 |
|
|
|
|
 |
C.E.Rogge,
W.Liu,
R.J.Kulmacz,
and
A.L.Tsai
(2009).
Peroxide-induced radical formation at TYR385 and TYR504 in human PGHS-1.
|
| |
J Inorg Biochem,
103,
912-922.
|
 |
|
|
|
|
 |
D.Raffa,
B.Maggio,
F.Plescia,
S.Cascioferro,
M.V.Raimondi,
S.Plescia,
and
M.G.Cusimano
(2009).
Pyrazolo[3,4-d]pyrimidine derivatives as COX-2 selective inhibitors: synthesis and molecular modelling studies.
|
| |
Arch Pharm (Weinheim),
342,
321-326.
|
 |
|
|
|
|
 |
P.Tosco,
and
L.Lazzarato
(2009).
Mechanistic insights into cyclooxygenase irreversible inactivation by aspirin.
|
| |
ChemMedChem,
4,
939-945.
|
 |
|
|
|
|
 |
B.J.Anderson
(2008).
Paracetamol (Acetaminophen): mechanisms of action.
|
| |
Paediatr Anaesth,
18,
915-921.
|
 |
|
|
|
|
 |
T.Saveria,
P.Kessler,
B.C.Jensen,
and
M.Parsons
(2007).
Characterization of glycosomal RING finger proteins of trypanosomatids.
|
| |
Exp Parasitol,
116,
14-24.
|
 |
|
|
|
|
 |
Y.C.Chen,
and
K.T.Chen
(2007).
Novel selective inhibitors of hydroxyxanthone derivatives for human cyclooxygenase-2.
|
| |
Acta Pharmacol Sin,
28,
2027-2032.
|
 |
|
|
|
|
 |
A.D.Kaposi,
J.M.Vanderkooi,
and
S.S.Stavrov
(2006).
Infrared absorption study of the heme pocket dynamics of carbonmonoxyheme proteins.
|
| |
Biophys J,
91,
4191-4200.
|
 |
|
|
|
|
 |
C.E.Rogge,
B.Ho,
W.Liu,
R.J.Kulmacz,
and
A.L.Tsai
(2006).
Role of Tyr348 in Tyr385 radical dynamics and cyclooxygenase inhibitor interactions in prostaglandin H synthase-2.
|
| |
Biochemistry,
45,
523-532.
|
 |
|
|
|
|
 |
K.E.Furse,
D.A.Pratt,
N.A.Porter,
and
T.P.Lybrand
(2006).
Molecular dynamics simulations of arachidonic acid complexes with COX-1 and COX-2: insights into equilibrium behavior.
|
| |
Biochemistry,
45,
3189-3205.
|
 |
|
|
|
|
 |
K.Gupta,
B.S.Selinsky,
and
P.J.Loll
(2006).
2.0 angstroms structure of prostaglandin H2 synthase-1 reconstituted with a manganese porphyrin cofactor.
|
| |
Acta Crystallogr D Biol Crystallogr,
62,
151-156.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.Bingham,
P.J.Beswick,
D.E.Blum,
N.M.Gray,
and
I.P.Chessell
(2006).
The role of the cylooxygenase pathway in nociception and pain.
|
| |
Semin Cell Dev Biol,
17,
544-554.
|
 |
|
|
|
|
 |
E.Munsterhjelm,
N.M.Munsterhjelm,
T.T.Niemi,
O.Ylikorkala,
P.J.Neuvonen,
and
P.H.Rosenberg
(2005).
Dose-dependent inhibition of platelet function by acetaminophen in healthy volunteers.
|
| |
Anesthesiology,
103,
712-717.
|
 |
|
|
|
|
 |
J.C.Wilson,
G.Wu,
A.L.Tsai,
and
G.J.Gerfen
(2005).
Determination of the structural environment of the tyrosyl radical in prostaglandin H2 synthase-1: a high frequency ENDOR/EPR study.
|
| |
J Am Chem Soc,
127,
1618-1619.
|
 |
|
|
|
|
 |
J.Lee,
A.J.Chubb,
E.Moman,
B.M.McLoughlin,
C.T.Sharkey,
J.G.Kelly,
K.B.Nolan,
M.Devocelle,
and
D.J.Fitzgerald
(2005).
Parallel synthesis and in vitro activity of novel anthranilic hydroxamate-based inhibitors of the prostaglandin H2 synthase peroxidase activity.
|
| |
Org Biomol Chem,
3,
3678-3685.
|
 |
|
|
|
|
 |
R.G.Huff,
E.Bayram,
H.Tan,
S.T.Knutson,
M.H.Knaggs,
A.B.Richon,
P.Santago,
and
J.S.Fetrow
(2005).
Chemical and structural diversity in cyclooxygenase protein active sites.
|
| |
Chem Biodivers,
2,
1533-1552.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

