 |
PDBsum entry 1il2

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Ligase/RNA
|
 |
|
Title:
|
 |
Crystal structure of the e. Coli aspartyl-tRNA synthetase:yeast trnaasp:aspartyl-adenylate complex
|
|
Structure:
|
 |
Aspartyl transfer RNA. Chain: c, d. Aspartyl-tRNA synthetase. Chain: a, b. Engineered: yes
|
|
Source:
|
 |
Saccharomyces cerevisiae. Baker's yeast. Organism_taxid: 4932. Escherichia coli. Organism_taxid: 562. Expressed in: escherichia coli. Expression_system_taxid: 562.
|
|
Biol. unit:
|
 |
Tetramer (from
)
|
|
Resolution:
|
 |
|
2.60Å
|
R-factor:
|
0.204
|
R-free:
|
0.257
|
|
|
Authors:
|
 |
L.Moulinier,S.Eiler,G.Eriani,J.Gangloff,J.C.Thierry,K.Gabriel, W.H.Mcclain,D.Moras
|
Key ref:

|
 |
L.Moulinier
et al.
(2001).
The structure of an AspRS-tRNA(Asp) complex reveals a tRNA-dependent control mechanism.
EMBO J,
20,
5290-5301.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
07-May-01
|
Release date:
|
28-Sep-01
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P21889
(SYD_ECOLI) -
Aspartate--tRNA ligase from Escherichia coli (strain K12)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
590 a.a.
585 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|

|

|
|
|
|
U-C-C-G-U-G-A-U-A-G-U-U-PSU-A-A-H2U-G-G-H2U-C-A-G-A-A-U-G-G-G-C-G-C-PSU-U-G-U-
75 bases
|
|
|
|
C-C-G-U-G-A-U-A-G-U-U-PSU-A-A-H2U-G-G-H2U-C-A-G-A-A-U-G-G-G-C-G-C-PSU-U-G-U-C-
70 bases
|
|

|
 |
 |
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.1.1.12
- aspartate--tRNA ligase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
tRNA(Asp) + L-aspartate + ATP = L-aspartyl-tRNA(Asp) + AMP + diphosphate
|
 |
 |
 |
 |
 |
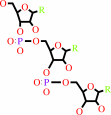 tRNA(Asp)
tRNA(Asp)
|
+
|
 L-aspartate
L-aspartate
|
+
|
 ATP
ATP
|
=
|
L-aspartyl-tRNA(Asp)
Bound ligand (Het Group name = )
matches with 74.19% similarity
|
+
|
 AMP
AMP
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
EMBO J
20:5290-5301
(2001)
|
|
PubMed id:
|
|
|
|

|
| |
|
The structure of an AspRS-tRNA(Asp) complex reveals a tRNA-dependent control mechanism.
|
|
L.Moulinier,
S.Eiler,
G.Eriani,
J.Gangloff,
J.C.Thierry,
K.Gabriel,
W.H.McClain,
D.Moras.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The 2.6 A resolution crystal structure of an inactive complex between yeast
tRNA(Asp) and Escherichia coli aspartyl-tRNA synthetase reveals the molecular
details of a tRNA-induced mechanism that controls the specificity of the
reaction. The dimer is asymmetric, with only one of the two bound tRNAs entering
the active site cleft of its subunit. However, the flipping loop, which controls
the proper positioning of the amino acid substrate, acts as a lid and prevents
the correct positioning of the terminal adenosine. The structure suggests that
the acceptor stem regulates the loop movement through sugar phosphate backbone-
protein interactions. Solution and cellular studies on mutant tRNAs confirm the
crucial role of the tRNA three-dimensional structure versus a specific
recognition of bases in the control mechanism.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Figure 3 (A) General view of the dimeric aspartyl-tRNA
synthetase from E.coli complexed with yeast tRNA^Asp and
aspartyl-adenylate. The tRNA^Asp molecules, colored in red and
yellow, are bound to one protein subunit shown in brown and
white, respectively. (B) AspRS surface buried by the tRNA in
monomer 1 (left) and monomer 2 (right) calculated and displayed
using GRASP (Nicholls and Honig, 1991). The surface is colored
according to a distance array between the two molecular
surfaces: distances <2.5 Å between the tRNA and the enzyme are
drawn in green, and distances between 2.5 and 3.5 Å are in
yellow. The interaction surfaces are highly similar for the
protein N-terminal domain in both monomers but vary through the
rest of the complex. (C) Ribbon representation of one AspRS
subunit in gray (monomer 1) of the heterologous complex with the
bound yeast tRNA^Asp in yellow. The E.coli tRNA^Asp as seen in
the cognate complex is drawn in blue after superposition of the
enzymes on their active sites. (D) Relative position of the two
tRNAs of the heterologous complex. Superposition was optimized
on the two subunit active sites. The tRNA^Asp from monomer 1 is
drawn in yellow, the tRNA from monomer 2 in red. (E) Comparison
of the tRNA molecule from monomer 2 of the heterologous complex
(red) and the free (uncomplexed) yeast tRNA^Asp (green) (Moras
et al., 1980). Figures 3, Figures 4, 5 were generated using the
Program SETOR (Evans, 1998).
|
 |
Figure 4.
Figure 4 Yeast tRNA^Asp acceptor stem in monomer 1 (A) and
monomer 2 (B) showing the U -G mismatches. The tRNA acceptor
stem in monomer 1 is bound to the enzymes and shows a regular
RNA conformation for the backbone dihedral angles  and and
 (gauche-/gauche+);
the distance between the phosphorus atoms of G68 and C69 is 5.6
Å. The tRNA molecule in monomer 2 shows no contact between the
acceptor stem and the protein. As a consequence, the dihedral
angles (gauche-/gauche+);
the distance between the phosphorus atoms of G68 and C69 is 5.6
Å. The tRNA molecule in monomer 2 shows no contact between the
acceptor stem and the protein. As a consequence, the dihedral
angles  and and
 are
trans/trans for G68, and the distance between the phosphorus
atoms of G68 and C69 is 6.6 Å. 'Accommodation' of U -G
mismatches has already been observed for the yeast AspRS
-tRNA^Asp complex (equivalent to tRNA 1) and for the free tRNA
(equivalent to tRNA 2). (C) Recognition of the discriminator
base G73 of yeast tRNA^Asp by the E.coli AspRS. The hydrogen
bonds between the protein and the nucleic acid are shown as
yellow dotted lines. They are similar to those observed in the
homologous E.coli AspRS -tRNA^Asp complex. are
trans/trans for G68, and the distance between the phosphorus
atoms of G68 and C69 is 6.6 Å. 'Accommodation' of U -G
mismatches has already been observed for the yeast AspRS
-tRNA^Asp complex (equivalent to tRNA 1) and for the free tRNA
(equivalent to tRNA 2). (C) Recognition of the discriminator
base G73 of yeast tRNA^Asp by the E.coli AspRS. The hydrogen
bonds between the protein and the nucleic acid are shown as
yellow dotted lines. They are similar to those observed in the
homologous E.coli AspRS -tRNA^Asp complex.
|
 |
|
|

|
| |
The above figures are
reprinted
from an Open Access publication published by Macmillan Publishers Ltd:
EMBO J
(2001,
20,
5290-5301)
copyright 2001.
|
|
| |
Figures were
selected
by the author.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
E.A.Merritt,
T.L.Arakaki,
E.T.Larson,
A.Kelley,
N.Mueller,
A.J.Napuli,
L.Zhang,
G.Deditta,
J.Luft,
C.L.Verlinde,
E.Fan,
F.Zucker,
F.S.Buckner,
W.C.Van Voorhis,
and
W.G.Hol
(2010).
Crystal structure of the aspartyl-tRNA synthetase from Entamoeba histolytica.
|
| |
Mol Biochem Parasitol,
169,
95.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
W.W.Navarre,
S.B.Zou,
H.Roy,
J.L.Xie,
A.Savchenko,
A.Singer,
E.Edvokimova,
L.R.Prost,
R.Kumar,
M.Ibba,
and
F.C.Fang
(2010).
PoxA, yjeK, and elongation factor P coordinately modulate virulence and drug resistance in Salmonella enterica.
|
| |
Mol Cell,
39,
209-221.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
T.Bour,
A.Akaddar,
B.Lorber,
S.Blais,
C.Balg,
E.Candolfi,
and
M.Frugier
(2009).
Plasmodial Aspartyl-tRNA Synthetases and Peculiarities in Plasmodium falciparum.
|
| |
J Biol Chem,
284,
18893-18903.
|
 |
|
|
|
|
 |
A.Minajigi,
and
C.S.Francklyn
(2008).
RNA-assisted catalysis in a protein enzyme: The 2'-hydroxyl of tRNA(Thr) A76 promotes aminoacylation by threonyl-tRNA synthetase.
|
| |
Proc Natl Acad Sci U S A,
105,
17748-17753.
|
 |
|
|
|
|
 |
C.S.Francklyn
(2008).
DNA polymerases and aminoacyl-tRNA synthetases: shared mechanisms for ensuring the fidelity of gene expression.
|
| |
Biochemistry,
47,
11695-11703.
|
 |
|
|
|
|
 |
D.Thompson,
C.Lazennec,
P.Plateau,
and
T.Simonson
(2008).
Probing electrostatic interactions and ligand binding in aspartyl-tRNA synthetase through site-directed mutagenesis and computer simulations.
|
| |
Proteins,
71,
1450-1460.
|
 |
|
|
|
|
 |
H.Xiao,
H.Murakami,
H.Suga,
and
A.R.Ferré-D'Amaré
(2008).
Structural basis of specific tRNA aminoacylation by a small in vitro selected ribozyme.
|
| |
Nature,
454,
358-361.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.Giegé
(2008).
Toward a more complete view of tRNA biology.
|
| |
Nat Struct Mol Biol,
15,
1007-1014.
|
 |
|
|
|
|
 |
S.An,
G.Barany,
and
K.Musier-Forsyth
(2008).
Evolution of acceptor stem tRNA recognition by class II prolyl-tRNA synthetase.
|
| |
Nucleic Acids Res,
36,
2514-2521.
|
 |
|
|
|
|
 |
D.Thompson,
C.Lazennec,
P.Plateau,
and
T.Simonson
(2007).
Ammonium scanning in an enzyme active site. The chiral specificity of aspartyl-tRNA synthetase.
|
| |
J Biol Chem,
282,
30856-30868.
|
 |
|
|
|
|
 |
E.C.Guth,
and
C.S.Francklyn
(2007).
Kinetic discrimination of tRNA identity by the conserved motif 2 loop of a class II aminoacyl-tRNA synthetase.
|
| |
Mol Cell,
25,
531-542.
|
 |
|
|
|
|
 |
I.A.Vasil'eva,
and
N.A.Moor
(2007).
Interaction of aminoacyl-tRNA synthetases with tRNA: general principles and distinguishing characteristics of the high-molecular-weight substrate recognition.
|
| |
Biochemistry (Mosc),
72,
247-263.
|
 |
|
|
|
|
 |
S.Kamtekar,
M.J.Hohn,
H.S.Park,
M.Schnitzbauer,
A.Sauerwald,
D.Söll,
and
T.A.Steitz
(2007).
Toward understanding phosphoseryl-tRNACys formation: the crystal structure of Methanococcus maripaludis phosphoseryl-tRNA synthetase.
|
| |
Proc Natl Acad Sci U S A,
104,
2620-2625.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.E.Rosen,
B.S.Brooks,
E.Guth,
C.S.Francklyn,
and
K.Musier-Forsyth
(2006).
Evolutionary conservation of a functionally important backbone phosphate group critical for aminoacylation of histidine tRNAs.
|
| |
RNA,
12,
1315-1322.
|
 |
|
|
|
|
 |
A.Fender,
C.Sauter,
M.Messmer,
J.Pütz,
R.Giegé,
C.Florentz,
and
M.Sissler
(2006).
Loss of a primordial identity element for a mammalian mitochondrial aminoacylation system.
|
| |
J Biol Chem,
281,
15980-15986.
|
 |
|
|
|
|
 |
A.Metlitskaya,
T.Kazakov,
A.Kommer,
O.Pavlova,
M.Praetorius-Ibba,
M.Ibba,
I.Krasheninnikov,
V.Kolb,
I.Khmel,
and
K.Severinov
(2006).
Aspartyl-tRNA synthetase is the target of peptide nucleotide antibiotic Microcin C.
|
| |
J Biol Chem,
281,
18033-18042.
|
 |
|
|
|
|
 |
D.Thompson,
P.Plateau,
and
T.Simonson
(2006).
Free-energy simulations and experiments reveal long-range electrostatic interactions and substrate-assisted specificity in an aminoacyl-tRNA synthetase.
|
| |
Chembiochem,
7,
337-344.
|
 |
|
|
|
|
 |
D.Thompson,
and
T.Simonson
(2006).
Molecular dynamics simulations show that bound Mg2+ contributes to amino acid and aminoacyl adenylate binding specificity in aspartyl-tRNA synthetase through long range electrostatic interactions.
|
| |
J Biol Chem,
281,
23792-23803.
|
 |
|
|
|
|
 |
S.J.Hughes,
J.A.Tanner,
A.D.Miller,
and
I.R.Gould
(2006).
Molecular dynamics simulations of LysRS: an asymmetric state.
|
| |
Proteins,
62,
649-662.
|
 |
|
|
|
|
 |
W.H.McClain
(2006).
Surprising contribution to aminoacylation and translation of non-Watson-Crick pairs in tRNA.
|
| |
Proc Natl Acad Sci U S A,
103,
4570-4575.
|
 |
|
|
|
|
 |
D.Lee,
and
W.H.McClain
(2004).
Aptamer redesigned tRNA is nonfunctional and degraded in cells.
|
| |
RNA,
10,
7.
|
 |
|
|
|
|
 |
F.Martin,
S.Barends,
and
G.Eriani
(2004).
Single amino acid changes in AspRS reveal alternative routes for expanding its tRNA repertoire in vivo.
|
| |
Nucleic Acids Res,
32,
4081-4089.
|
 |
|
|
|
|
 |
P.Auffinger,
L.Bielecki,
and
E.Westhof
(2004).
Anion binding to nucleic acids.
|
| |
Structure,
12,
379-388.
|
 |
|
|
|
|
 |
A.Brevet,
J.Chen,
S.Commans,
C.Lazennec,
S.Blanquet,
and
P.Plateau
(2003).
Anticodon recognition in evolution: switching tRNA specificity of an aminoacyl-tRNA synthetase by site-directed peptide transplantation.
|
| |
J Biol Chem,
278,
30927-30935.
|
 |
|
|
|
|
 |
A.R.Ferré-D'Amaré
(2003).
RNA-modifying enzymes.
|
| |
Curr Opin Struct Biol,
13,
49-55.
|
 |
|
|
|
|
 |
B.Min,
M.Kitabatake,
C.Polycarpo,
J.Pelaschier,
G.Raczniak,
B.Ruan,
H.Kobayashi,
S.Namgoong,
and
D.Söll
(2003).
Protein synthesis in Escherichia coli with mischarged tRNA.
|
| |
J Bacteriol,
185,
3524-3526.
|
 |
|
|
|
|
 |
C.Charron,
H.Roy,
M.Blaise,
R.Giegé,
and
D.Kern
(2003).
Non-discriminating and discriminating aspartyl-tRNA synthetases differ in the anticodon-binding domain.
|
| |
EMBO J,
22,
1632-1643.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
H.Choi,
K.Gabriel,
J.Schneider,
S.Otten,
and
W.H.McClain
(2003).
Recognition of acceptor-stem structure of tRNA(Asp) by Escherichia coli aspartyl-tRNA synthetase.
|
| |
RNA,
9,
386-393.
|
 |
|
|
|
|
 |
H.Roy,
H.D.Becker,
J.Reinbolt,
and
D.Kern
(2003).
When contemporary aminoacyl-tRNA synthetases invent their cognate amino acid metabolism.
|
| |
Proc Natl Acad Sci U S A,
100,
9837-9842.
|
 |
|
|
|
|
 |
R.Geslain,
F.Martin,
A.Camasses,
and
G.Eriani
(2003).
A yeast knockout strain to discriminate between active and inactive tRNA molecules.
|
| |
Nucleic Acids Res,
31,
4729-4737.
|
 |
|
|
|
|
 |
A.Torres-Larios,
A.C.Dock-Bregeon,
P.Romby,
B.Rees,
R.Sankaranarayanan,
J.Caillet,
M.Springer,
C.Ehresmann,
B.Ehresmann,
and
D.Moras
(2002).
Structural basis of translational control by Escherichia coli threonyl tRNA synthetase.
|
| |
Nat Struct Biol,
9,
343-347.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.Francklyn,
J.J.Perona,
J.Puetz,
and
Y.M.Hou
(2002).
Aminoacyl-tRNA synthetases: versatile players in the changing theater of translation.
|
| |
RNA,
8,
1363-1372.
|
 |
|
|
|
|
 |
F.Walter,
J.Pütz,
R.Giegé,
and
E.Westhof
(2002).
Binding of tobramycin leads to conformational changes in yeast tRNA(Asp) and inhibition of aminoacylation.
|
| |
EMBO J,
21,
760-768.
|
 |
|
|
|
|
 |
I.Gruic-Sovulj,
I.Landeka,
D.Söll,
and
I.Weygand-Durasevic
(2002).
tRNA-dependent amino acid discrimination by yeast seryl-tRNA synthetase.
|
| |
Eur J Biochem,
269,
5271-5279.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

