 |
PDBsum entry 1gts

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.1.1.18
- glutamine--tRNA ligase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
tRNA(Gln) + L-glutamine + ATP = L-glutaminyl-tRNA(Gln) + AMP + diphosphate
|
 |
 |
 |
 |
 |
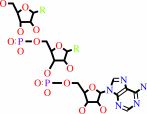 tRNA(Gln)
tRNA(Gln)
|
+
|
 L-glutamine
L-glutamine
|
+
|
 ATP
ATP
|
=
|
L-glutaminyl-tRNA(Gln)
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
 AMP
AMP
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
32:8758-8771
(1993)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural basis for transfer RNA aminoacylation by Escherichia coli glutaminyl-tRNA synthetase.
|
|
J.J.Perona,
M.A.Rould,
T.A.Steitz.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The structure of Escherichia coli glutaminyl-tRNA synthetase complexed with
tRNA2Gln and ATP refined at 2.5-A resolution reveals structural details of the
catalytic center and allows description of the specific roles of individual
amino acid residues in substrate binding and catalysis. The reactive moieties of
the ATP and tRNA substrates are positioned within hydrogen-bonding distance of
each other. Model-building has been used to position the glutamine substrate in
an adjacent cavity with its reactive carboxylate adjacent to the alpha-phosphate
of ATP; the interactions of the carboxyamide side chain suggest a structural
rationale for the way in which the enzyme discriminates against glutamate. The
binding site for a manganese ion has also been identified bridging the beta- and
gamma-phosphates of the ATP. The well-known HIGH and KMSKS sequence motifs
interact directly with each other as well as with the ATP, providing a
structural rationale for their simultaneous conservation in all class I
synthetases. The KMSKS loop adopts a well-ordered and catalytically productive
conformation as a consequence of interactions made with the proximal beta-barrel
domain. While there are no protein side chains near the reaction site that might
function in acid-base catalysis, the side chains of two residues, His43 and
Lys270, are positioned to assist in stabilizing the expected pentacovalent
intermediate at the alpha-phosphate. Transfer of glutamine to the 3'-terminal
tRNA ribose may well proceed by intramolecular catalysis involving proton
abstraction by a phosphate oxygen atom of glutaminyl adenylate. Catalytic
competence of the crystalline enzyme is directly shown by its ability to
hydrolyze ATP and release pyrophosphate when crystals of the ternary complex are
soaked in mother liquor containing glutamine.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.Palencia,
T.Crépin,
M.T.Vu,
T.L.Lincecum,
S.A.Martinis,
and
S.Cusack
(2012).
Structural dynamics of the aminoacylation and proofreading functional cycle of bacterial leucyl-tRNA synthetase.
|
| |
Nat Struct Mol Biol,
19,
677-684.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Sethi,
J.Eargle,
A.A.Black,
and
Z.Luthey-Schulten
(2009).
Dynamical networks in tRNA:protein complexes.
|
| |
Proc Natl Acad Sci U S A,
106,
6620-6625.
|
 |
|
|
|
|
 |
E.M.Corigliano,
and
J.J.Perona
(2009).
Architectural underpinnings of the genetic code for glutamine.
|
| |
Biochemistry,
48,
676-687.
|
 |
|
|
|
|
 |
F.Fan,
and
J.S.Blanchard
(2009).
Toward the catalytic mechanism of a cysteine ligase (MshC) from Mycobacterium smegmatis: an enzyme involved in the biosynthetic pathway of mycothiol.
|
| |
Biochemistry,
48,
7150-7159.
|
 |
|
|
|
|
 |
J.W.Wong,
J.P.McRedmond,
and
G.Cagney
(2009).
Activity profiling of platelets by chemical proteomics.
|
| |
Proteomics,
9,
40-50.
|
 |
|
|
|
|
 |
T.T.Doan,
S.Natarajan,
H.Kim,
Y.J.Ahn,
J.G.Kim,
B.M.Lee,
and
L.W.Kang
(2009).
Cloning, expression, crystallization and preliminary X-ray crystallographic analysis of glutamyl-tRNA synthetase (Xoo1504) from Xanthomonas oryzae pv. oryzae.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
65,
51-54.
|
 |
|
|
|
|
 |
A.Minajigi,
and
C.S.Francklyn
(2008).
RNA-assisted catalysis in a protein enzyme: The 2'-hydroxyl of tRNA(Thr) A76 promotes aminoacylation by threonyl-tRNA synthetase.
|
| |
Proc Natl Acad Sci U S A,
105,
17748-17753.
|
 |
|
|
|
|
 |
L.W.Tremblay,
F.Fan,
M.W.Vetting,
and
J.S.Blanchard
(2008).
The 1.6 A crystal structure of Mycobacterium smegmatis MshC: the penultimate enzyme in the mycothiol biosynthetic pathway.
|
| |
Biochemistry,
47,
13326-13335.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
I.A.Vasil'eva,
and
N.A.Moor
(2007).
Interaction of aminoacyl-tRNA synthetases with tRNA: general principles and distinguishing characteristics of the high-molecular-weight substrate recognition.
|
| |
Biochemistry (Mosc),
72,
247-263.
|
 |
|
|
|
|
 |
R.Sathyapriya,
and
S.Vishveshwara
(2007).
Structure networks of E. coli glutaminyl-tRNA synthetase: effects of ligand binding.
|
| |
Proteins,
68,
541-550.
|
 |
|
|
|
|
 |
S.W.Lue,
and
S.O.Kelley
(2007).
A single residue in leucyl-tRNA synthetase affecting amino acid specificity and tRNA aminoacylation.
|
| |
Biochemistry,
46,
4466-4472.
|
 |
|
|
|
|
 |
J.S.Weinger,
and
S.A.Strobel
(2006).
Participation of the tRNA A76 hydroxyl groups throughout translation.
|
| |
Biochemistry,
45,
5939-5948.
|
 |
|
|
|
|
 |
N.T.Uter,
and
J.J.Perona
(2006).
Active-site assembly in glutaminyl-tRNA synthetase by tRNA-mediated induced fit.
|
| |
Biochemistry,
45,
6858-6865.
|
 |
|
|
|
|
 |
I.Gruic-Sovulj,
N.Uter,
T.Bullock,
and
J.J.Perona
(2005).
tRNA-dependent aminoacyl-adenylate hydrolysis by a nonediting class I aminoacyl-tRNA synthetase.
|
| |
J Biol Chem,
280,
23978-23986.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
N.T.Uter,
I.Gruic-Sovulj,
and
J.J.Perona
(2005).
Amino acid-dependent transfer RNA affinity in a class I aminoacyl-tRNA synthetase.
|
| |
J Biol Chem,
280,
23966-23977.
|
 |
|
|
|
|
 |
V.K.Morris,
and
T.Izard
(2004).
Substrate-induced asymmetry and channel closure revealed by the apoenzyme structure of Mycobacterium tuberculosis phosphopantetheine adenylyltransferase.
|
| |
Protein Sci,
13,
2547-2552.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.Parschat,
B.Hauer,
R.Kappl,
R.Kraft,
J.Huttermann,
and
S.Fetzner
(2003).
Gene cluster of Arthrobacter ilicis Ru61a involved in the degradation of quinaldine to anthranilate: characterization and functional expression of the quinaldine 4-oxidase qoxLMS genes.
|
| |
J Biol Chem,
278,
27483-27494.
|
 |
|
|
|
|
 |
L.D.Sherlin,
and
J.J.Perona
(2003).
tRNA-dependent active site assembly in a class I aminoacyl-tRNA synthetase.
|
| |
Structure,
11,
591-603.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.Geslain,
G.Bey,
J.Cavarelli,
and
G.Eriani
(2003).
Limited set of amino acid residues in a class Ia aminoacyl-tRNA synthetase is crucial for tRNA binding.
|
| |
Biochemistry,
42,
15092-15101.
|
 |
|
|
|
|
 |
S.Sekine,
O.Nureki,
D.Y.Dubois,
S.Bernier,
R.Chênevert,
J.Lapointe,
D.G.Vassylyev,
and
S.Yokoyama
(2003).
ATP binding by glutamyl-tRNA synthetase is switched to the productive mode by tRNA binding.
|
| |
EMBO J,
22,
676-688.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.Izard
(2003).
A novel adenylate binding site confers phosphopantetheine adenylyltransferase interactions with coenzyme A.
|
| |
J Bacteriol,
185,
4074-4080.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
V.Saridakis,
and
E.F.Pai
(2003).
Mutational, structural, and kinetic studies of the ATP-binding site of Methanobacterium thermoautotrophicum nicotinamide mononucleotide adenylyltransferase.
|
| |
J Biol Chem,
278,
34356-34363.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.D.Wolfson,
and
O.C.Uhlenbeck
(2002).
Modulation of tRNAAla identity by inorganic pyrophosphatase.
|
| |
Proc Natl Acad Sci U S A,
99,
5965-5970.
|
 |
|
|
|
|
 |
A.Yaremchuk,
I.Kriklivyi,
M.Tukalo,
and
S.Cusack
(2002).
Class I tyrosyl-tRNA synthetase has a class II mode of cognate tRNA recognition.
|
| |
EMBO J,
21,
3829-3840.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.J.Newberry,
Y.M.Hou,
and
J.J.Perona
(2002).
Structural origins of amino acid selection without editing by cysteinyl-tRNA synthetase.
|
| |
EMBO J,
21,
2778-2787.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Garavaglia,
I.D'Angelo,
M.Emanuelli,
F.Carnevali,
F.Pierella,
G.Magni,
and
M.Rizzi
(2002).
Structure of human NMN adenylyltransferase. A key nuclear enzyme for NAD homeostasis.
|
| |
J Biol Chem,
277,
8524-8530.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Pingoud,
and
A.Jeltsch
(2001).
Structure and function of type II restriction endonucleases.
|
| |
Nucleic Acids Res,
29,
3705-3727.
|
 |
|
|
|
|
 |
F.von Delft,
A.Lewendon,
V.Dhanaraj,
T.L.Blundell,
C.Abell,
and
A.G.Smith
(2001).
The crystal structure of E. coli pantothenate synthetase confirms it as a member of the cytidylyltransferase superfamily.
|
| |
Structure,
9,
439-450.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.J.Salama,
I.Donaldson,
and
C.W.Hogue
(2001).
Automatic annotation of BIND molecular interactions from three-dimensional structures.
|
| |
Biopolymers,
61,
111-120.
|
 |
|
|
|
|
 |
T.C.Ullrich,
M.Blaesse,
and
R.Huber
(2001).
Crystal structure of ATP sulfurylase from Saccharomyces cerevisiae, a key enzyme in sulfate activation.
|
| |
EMBO J,
20,
316-329.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
I.D'Angelo,
N.Raffaelli,
V.Dabusti,
T.Lorenzi,
G.Magni,
and
M.Rizzi
(2000).
Structure of nicotinamide mononucleotide adenylyltransferase: a key enzyme in NAD(+) biosynthesis.
|
| |
Structure,
8,
993.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.A.Denessiouk,
and
M.S.Johnson
(2000).
When fold is not important: a common structural framework for adenine and AMP binding in 12 unrelated protein families.
|
| |
Proteins,
38,
310-326.
|
 |
|
|
|
|
 |
M.Ibba,
and
D.Soll
(2000).
Aminoacyl-tRNA synthesis.
|
| |
Annu Rev Biochem,
69,
617-650.
|
 |
|
|
|
|
 |
W.Dall'Acqua,
and
P.Carter
(2000).
Substrate-assisted catalysis: molecular basis and biological significance.
|
| |
Protein Sci,
9,
1-9.
|
 |
|
|
|
|
 |
A.Geerlof,
A.Lewendon,
and
W.V.Shaw
(1999).
Purification and characterization of phosphopantetheine adenylyltransferase from Escherichia coli.
|
| |
J Biol Chem,
274,
27105-27111.
|
 |
|
|
|
|
 |
C.H.Weber,
Y.S.Park,
S.Sanker,
C.Kent,
and
M.L.Ludwig
(1999).
A prototypical cytidylyltransferase: CTP:glycerol-3-phosphate cytidylyltransferase from bacillus subtilis.
|
| |
Structure,
7,
1113-1124.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
L.F.Silvian,
J.Wang,
and
T.A.Steitz
(1999).
Insights into editing from an ile-tRNA synthetase structure with tRNAile and mupirocin.
|
| |
Science,
285,
1074-1077.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.W.Alexander,
and
P.Schimmel
(1999).
Evidence for breaking domain-domain functional communication in a synthetase-tRNA complex.
|
| |
Biochemistry,
38,
16359-16365.
|
 |
|
|
|
|
 |
T.Izard,
and
A.Geerlof
(1999).
The crystal structure of a novel bacterial adenylyltransferase reveals half of sites reactivity.
|
| |
EMBO J,
18,
2021-2030.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
F.Agou,
S.Quevillon,
P.Kerjan,
and
M.Mirande
(1998).
Switching the amino acid specificity of an aminoacyl-tRNA synthetase.
|
| |
Biochemistry,
37,
11309-11314.
|
 |
|
|
|
|
 |
F.Stahl,
W.Wende,
A.Jeltsch,
and
A.Pingoud
(1998).
The mechanism of DNA cleavage by the type II restriction enzyme EcoRV: Asp36 is not directly involved in DNA cleavage but serves to couple indirect readout to catalysis.
|
| |
Biol Chem,
379,
467-473.
|
 |
|
|
|
|
 |
V.L.Rath,
L.F.Silvian,
B.Beijer,
B.S.Sproat,
and
T.A.Steitz
(1998).
How glutaminyl-tRNA synthetase selects glutamine.
|
| |
Structure,
6,
439-449.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Aberg,
A.Yaremchuk,
M.Tukalo,
B.Rasmussen,
and
S.Cusack
(1997).
Crystal structure analysis of the activation of histidine by Thermus thermophilus histidyl-tRNA synthetase.
|
| |
Biochemistry,
36,
3084-3094.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Pingoud,
and
A.Jeltsch
(1997).
Recognition and cleavage of DNA by type-II restriction endonucleases.
|
| |
Eur J Biochem,
246,
1.
|
 |
|
|
|
|
 |
J.J.Tesmer,
R.K.Sunahara,
A.G.Gilman,
and
S.R.Sprang
(1997).
Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsalpha.GTPgammaS.
|
| |
Science,
278,
1907-1916.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.J.Thiele,
M.Podda,
and
L.Packer
(1997).
Tropospheric ozone: an emerging environmental stress to skin.
|
| |
Biol Chem,
378,
1299-1305.
|
 |
|
|
|
|
 |
J.L.Riechmann,
and
E.M.Meyerowitz
(1997).
MADS domain proteins in plant development.
|
| |
Biol Chem,
378,
1079-1101.
|
 |
|
|
|
|
 |
M.Sissler,
G.Eriani,
F.Martin,
R.Giegé,
and
C.Florentz
(1997).
Mirror image alternative interaction patterns of the same tRNA with either class I arginyl-tRNA synthetase or class II aspartyl-tRNA synthetase.
|
| |
Nucleic Acids Res,
25,
4899-4906.
|
 |
|
|
|
|
 |
N.Murali,
Y.Lin,
Y.Mechulam,
P.Plateau,
and
B.D.Rao
(1997).
Adenosine conformations of nucleotides bound to methionyl tRNA synthetase by transferred nuclear Overhauser effect spectroscopy.
|
| |
Biophys J,
72,
2275-2284.
|
 |
|
|
|
|
 |
S.Cusack
(1997).
Aminoacyl-tRNA synthetases.
|
| |
Curr Opin Struct Biol,
7,
881-889.
|
 |
|
|
|
|
 |
Y.Goldgur,
L.Mosyak,
L.Reshetnikova,
V.Ankilova,
O.Lavrik,
S.Khodyreva,
and
M.Safro
(1997).
The crystal structure of phenylalanyl-tRNA synthetase from thermus thermophilus complexed with cognate tRNAPhe.
|
| |
Structure,
5,
59-68.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
Y.S.Park,
P.Gee,
S.Sanker,
E.J.Schurter,
E.R.Zuiderweg,
and
C.Kent
(1997).
Identification of functional conserved residues of CTP:glycerol-3-phosphate cytidylyltransferase. Role of histidines in the conserved HXGH in catalysis.
|
| |
J Biol Chem,
272,
15161-15166.
|
 |
|
|
|
|
 |
D.P.Veitch,
and
R.B.Cornell
(1996).
Substitution of serine for glycine-91 in the HXGH motif of CTP:phosphocholine cytidylyltransferase implicates this motif in CTP binding.
|
| |
Biochemistry,
35,
10743-10750.
|
 |
|
|
|
|
 |
E.Conti,
N.P.Franks,
and
P.Brick
(1996).
Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes.
|
| |
Structure,
4,
287-298.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.A.Moore,
A.Chen,
M.Yan,
A.P.Hurlburt,
and
C.D.Poulter
(1996).
Identification of the gltX gene encoding glutamyl-tRNA synthetase from Methanobacterium thermoautotrophicum.
|
| |
Biochim Biophys Acta,
1305,
113-116.
|
 |
|
|
|
|
 |
J.G.Arnez,
and
T.A.Steitz
(1996).
Crystal structures of three misacylating mutants of Escherichia coli glutaminyl-tRNA synthetase complexed with tRNA(Gln) and ATP.
|
| |
Biochemistry,
35,
14725-14733.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.J.Tesmer,
T.J.Klem,
M.L.Deras,
V.J.Davisson,
and
J.L.Smith
(1996).
The crystal structure of GMP synthetase reveals a novel catalytic triad and is a structural paradigm for two enzyme families.
|
| |
Nat Struct Biol,
3,
74-86.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.A.LeCuyer,
L.S.Behlen,
and
O.C.Uhlenbeck
(1996).
Mutagenesis of a stacking contact in the MS2 coat protein-RNA complex.
|
| |
EMBO J,
15,
6847-6853.
|
 |
|
|
|
|
 |
K.Breitschopf,
and
H.J.Gross
(1996).
The discriminator bases G73 in human tRNA(Ser) and A73 in tRNA(Leu) have significantly different roles in the recognition of aminoacyl-tRNA synthetases.
|
| |
Nucleic Acids Res,
24,
405-410.
|
 |
|
|
|
|
 |
K.W.Hong,
M.Ibba,
I.Weygand-Durasevic,
M.J.Rogers,
H.U.Thomann,
and
D.Söll
(1996).
Transfer RNA-dependent cognate amino acid recognition by an aminoacyl-tRNA synthetase.
|
| |
EMBO J,
15,
1983-1991.
|
 |
|
|
|
|
 |
M.Ibba,
K.W.Hong,
J.M.Sherman,
S.Sever,
and
D.Söll
(1996).
Interactions between tRNA identity nucleotides and their recognition sites in glutaminyl-tRNA synthetase determine the cognate amino acid affinity of the enzyme.
|
| |
Proc Natl Acad Sci U S A,
93,
6953-6958.
|
 |
|
|
|
|
 |
M.Rizzi,
C.Nessi,
A.Mattevi,
A.Coda,
M.Bolognesi,
and
A.Galizzi
(1996).
Crystal structure of NH3-dependent NAD+ synthetase from Bacillus subtilis.
|
| |
EMBO J,
15,
5125-5134.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.K.Airas
(1996).
Differences in the magnesium dependences of the class I and class II aminoacyl-tRNA synthetases from Escherichia coli.
|
| |
Eur J Biochem,
240,
223-231.
|
 |
|
|
|
|
 |
S.Cusack,
A.Yaremchuk,
and
M.Tukalo
(1996).
The crystal structure of the ternary complex of T.thermophilus seryl-tRNA synthetase with tRNA(Ser) and a seryl-adenylate analogue reveals a conformational switch in the active site.
|
| |
EMBO J,
15,
2834-2842.
|
 |
|
|
|
|
 |
Y.Gagnon,
L.Lacoste,
N.Champagne,
and
J.Lapointe
(1996).
Widespread use of the glu-tRNAGln transamidation pathway among bacteria. A member of the alpha purple bacteria lacks glutaminyl-trna synthetase.
|
| |
J Biol Chem,
271,
14856-14863.
|
 |
|
|
|
|
 |
E.Schmitt,
M.Panvert,
S.Blanquet,
and
Y.Mechulam
(1995).
Transition state stabilization by the 'high' motif of class I aminoacyl-tRNA synthetases: the case of Escherichia coli methionyl-tRNA synthetase.
|
| |
Nucleic Acids Res,
23,
4793-4798.
|
 |
|
|
|
|
 |
H.Belrhali,
A.Yaremchuk,
M.Tukalo,
C.Berthet-Colominas,
B.Rasmussen,
P.Bösecke,
O.Diat,
and
S.Cusack
(1995).
The structural basis for seryl-adenylate and Ap4A synthesis by seryl-tRNA synthetase.
|
| |
Structure,
3,
341-352.
|
 |
|
|
|
|
 |
M.Delarue
(1995).
Aminoacyl-tRNA synthetases.
|
| |
Curr Opin Struct Biol,
5,
48-55.
|
 |
|
|
|
|
 |
P.Bork,
L.Holm,
E.V.Koonin,
and
C.Sander
(1995).
The cytidylyltransferase superfamily: identification of the nucleotide-binding site and fold prediction.
|
| |
Proteins,
22,
259-266.
|
 |
|
|
|
|
 |
S.Cusack
(1995).
Eleven down and nine to go.
|
| |
Nat Struct Biol,
2,
824-831.
|
 |
|
|
|
|
 |
S.Doublié,
G.Bricogne,
C.Gilmore,
and
C.W.Carter
(1995).
Tryptophanyl-tRNA synthetase crystal structure reveals an unexpected homology to tyrosyl-tRNA synthetase.
|
| |
Structure,
3,
17-31.
|
 |
|
|
|
|
 |
T.Schweins,
M.Geyer,
K.Scheffzek,
A.Warshel,
H.R.Kalbitzer,
and
A.Wittinghofer
(1995).
Substrate-assisted catalysis as a mechanism for GTP hydrolysis of p21ras and other GTP-binding proteins.
|
| |
Nat Struct Biol,
2,
36-44.
|
 |
|
|
|
|
 |
J.Cavarelli,
G.Eriani,
B.Rees,
M.Ruff,
M.Boeglin,
A.Mitschler,
F.Martin,
J.Gangloff,
J.C.Thierry,
and
D.Moras
(1994).
The active site of yeast aspartyl-tRNA synthetase: structural and functional aspects of the aminoacylation reaction.
|
| |
EMBO J,
13,
327-337.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.Delarue,
A.Poterszman,
S.Nikonov,
M.Garber,
D.Moras,
and
J.C.Thierry
(1994).
Crystal structure of a prokaryotic aspartyl tRNA-synthetase.
|
| |
EMBO J,
13,
3219-3229.
|
 |
|
|
|
|
 |
M.J.Rogers,
T.Adachi,
H.Inokuchi,
and
D.Söll
(1994).
Functional communication in the recognition of tRNA by Escherichia coli glutaminyl-tRNA synthetase.
|
| |
Proc Natl Acad Sci U S A,
91,
291-295.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

