Ferredoxin:thioredoxin reductase
Ferredoxin:thioredoxin reductase (FTR) transfers the light-generated redox signal received by the chloroplast [Fe2S2]2+,+ ferredoxin (Fdx) to thioredoxins (Trxs), as part of a redox regulatory system controlling the activity of a wide range of oxygenic photosynthesis enzymes in response to light. The active site consists of a [Fe4S4] cluster and an an adjacent redox-active disulphide. FTR converts two light-generated one-electron signals to one two-electron thiol signal which is then transmitted via dithiol/disulphide interchange reactions to specific enzymes which are critical to the regulation of the Calvin cycle.
Phylogenetic analyses of genomic sequences revealed that the catalytic subunit of FTR originated in microaerophilic bacteria where it may have functioned in regulating CO2 fixation. Also, FTR may have been acquired by later-evolving species via horizontal gene transfer. FTR-like enzymes, for example FDR, with structural and functional diversity have evolved to meet ecological needs.
Reference Protein and Structure
- Sequence
-
Q55389
 (1.8.7.2)
(1.8.7.2)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Synechocystis sp. PCC 6803 substr. Kazusa (Bacteria)

- PDB
-
1dj7
- CRYSTAL STRUCTURE OF FERREDOXIN THIOREDOXIN REDUCTASE
(1.6 Å)



- Catalytic CATH Domains
-
3.90.460.10
 (see all for 1dj7)
(see all for 1dj7)
- Cofactors
- Tetra-mu3-sulfido-tetrairon (1)
Enzyme Mechanism
Introduction
One-electron reduction mechanism, formally viewed as two-electron reduction of the disulphide with concurrent one-electron oxidation of the cluster due to coordination of an additional cysteinate ligand. This anchors one of the active-site thiol ligands via cluster coordination while freeing the other thiol for nucleophilic attack of the Trx disulphide to form an FTR/Trx heterodisulphide intermediate. A subsequent one-electron reduction reduces the cluster to [Fe4S4]2+ and releases the electron-transfer thiol to reform the active-site disulphide with concomitant cleavage of the heterodisulphide and formation of the reduced dithiol form of Trx. Note, this mechanism is analogous to that followed by an FTR-like enzyme "FDR".
Catalytic Residues Roles
| UniProt | PDB* (1dj7) | ||
| His87 | His86A | His86 is proposed to play a functional role in protonation/deprotonation of the cluster-interacting thiol and anchoring the cluster interacting thiol in close proximity to the cluster in the two-electron-reduced intermediate. | electrostatic stabiliser |
| Cys88 | Cys87A | Cleavage of heterosulphide occurs via reductive release of Cys87 and subsequent nucleophilic attack of the heterodisulphide by Cys87, resulting in cleavage of the heterosulphide and reformation of the active site disulphide. | nucleofuge, metal ligand, nucleophile, activator, covalent catalysis, electrofuge |
| Cys58 | Cys57A | Interchange thiol responsible for attacking the Trx disulphide. | metal ligand, nucleofuge, nucleophile |
| Cys86, Cys56, Cys77, Cys75 | Cys85A, Cys55A, Cys76A, Cys74A | Attached to [Fe4S4]3+/2+ cluster. | metal ligand |
Chemical Components
substitution (not covered by the Ingold mechanisms), coordination to a metal ion, cofactor used, electron transfer, bimolecular nucleophilic substitution, intermediate formation, enzyme-substrate complex formation, proton transfer, native state of cofactor regenerated, decoordination from a metal ion, enzyme-substrate complex cleavage, intermediate terminated, native state of enzyme regenerated, overall product formedReferences
- Walters EM et al. (2005), J Am Chem Soc, 127, 9612-9624. Spectroscopic characterization of site-specific [Fe(4)S(4)] cluster chemistry in ferredoxin:thioredoxin reductase: implications for the catalytic mechanism. DOI:10.1021/ja051909q. PMID:15984889.
- Kumar AK et al. (2015), Biochemistry, 54, 3122-3128. Structural and Biochemical Characterization of a Ferredoxin:Thioredoxin Reductase-like Enzyme from Methanosarcina acetivorans. DOI:10.1021/acs.biochem.5b00137. PMID:25915695.
- Walters EM et al. (2009), Biochemistry, 48, 1016-1024. Role of histidine-86 in the catalytic mechanism of ferredoxin:thioredoxin reductase. DOI:10.1021/bi802074p. PMID:19132843.
- Walters EM et al. (2004), Photosynth Res, 79, 249-264. Ferredoxin:thioredoxin Reductase: Disulfide Reduction Catalyzed via Novel Site-specific [4Fe-4S] Cluster Chemistry. DOI:10.1023/B:PRES.0000017195.05870.61. PMID:16328791.
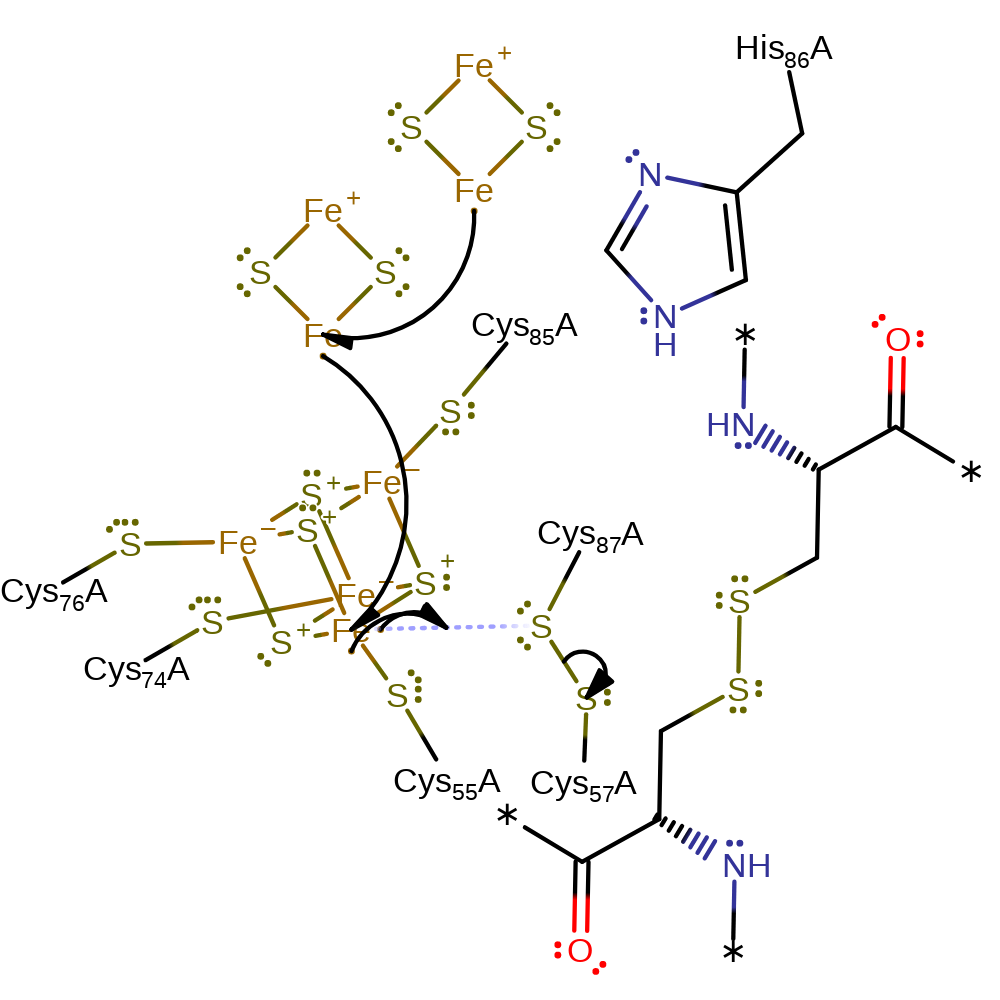
Step 1. The interaction between the cluster and Cys87 promotes charge build-up at the unique Fe site in the resting enzyme, priming the active site for one-electron reduction from ferredoxin leading to cleavage of the disulphide and attachment of Cys87 to the cluster. Overall one-electron oxidation of the cluster to [Fe4S4]3+.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys87A | activator, covalent catalysis, metal ligand |
| Cys85A | metal ligand |
| Cys55A | metal ligand |
| Cys76A | metal ligand |
| Cys74A | metal ligand |
| Cys57A | nucleofuge |
| His86A | electrostatic stabiliser |
| Cys87A | electrofuge |
Chemical Components
substitution (not covered by the Ingold mechanisms), coordination to a metal ion, cofactor used, electron transfer
Step 2. Cys57 performs a nucleophilic attack on the thioredoxin disulphide to form an FTR/Trx heterodisulphide intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys85A | metal ligand |
| Cys87A | metal ligand |
| Cys55A | metal ligand |
| Cys76A | metal ligand |
| Cys74A | metal ligand |
| His86A | electrostatic stabiliser |
| Cys57A | nucleophile |
Chemical Components
ingold: bimolecular nucleophilic substitution, intermediate formation, enzyme-substrate complex formationCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His86A | electrostatic stabiliser |
| Cys85A | metal ligand |
| Cys87A | metal ligand |
| Cys55A | metal ligand |
| Cys76A | metal ligand |
| Cys74A | metal ligand |
Chemical Components
proton transfer
Step 4. One-electron reduction of the cluster to [Fe4S4]2+ and release of Cys87.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys87A | nucleofuge |
| Cys85A | metal ligand |
| Cys87A | metal ligand |
| Cys57A | metal ligand |
| Cys76A | metal ligand |
| Cys74A | metal ligand |
Chemical Components
cofactor used, native state of cofactor regenerated, decoordination from a metal ion, electron transfer
Step 5. Cys87 reforms the active site disulphide with concomitant cleavage of the heterodisulphide intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys87A | nucleophile |
| Cys55A | electrofuge, electrophile |
| Cys85A | metal ligand |
| Cys57A | metal ligand |
| Cys76A | metal ligand |
| Cys74A | metal ligand |
Chemical Components
enzyme-substrate complex cleavage, intermediate terminated, native state of enzyme regenerated, ingold: bimolecular nucleophilic substitutionCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys85A | metal ligand |
| Cys55A | metal ligand |
| Cys76A | metal ligand |
| Cys74A | metal ligand |
Chemical Components
proton transfer, overall product formedIntroduction
In an alternative and equally viable mechanism, FTR is reduced by two electrons prior to interaction with Trx. The first single-electron-reduction forms a transient intermediate which is then further reduced to yield the two-electron-reduced species. The strong H-bonding interaction between formed by Cys87 frees Cys57 for nucleophilic attack of Trx. His86 is proposed to play a role in stabilising the two-electron intermediate via interaction with Cys87. This mechanism is very similar to that found in the more extensively studied nucleotide-dependent disulphide reductases.
Catalytic Residues Roles
| UniProt | PDB* (1dj7) | ||
| His87 | His86A | His86 is proposed to play a functional role in protonation/deprotonation of the cluster-interacting thiol and anchoring the cluster interacting thiol in close proximity to the cluster in the two-electron-reduced intermediate. | electrostatic stabiliser, proton donor |
| Cys88 | Cys87A | Cleavage of heterosulphide occurs via nucleophilic attack of the heterodisulphide by Cys87, resulting in cleavage of the heterosulphide and reformation of the active site disulphide. | hydrogen bond donor, metal ligand, proton acceptor, proton donor, covalent catalysis, electrofuge, electrophile |
| Cys58 | Cys57A | Interchange thiol responsible for attacking the Trx disulphide. | nucleophile, proton acceptor, proton donor, electrofuge, electrophile |
| Cys86, Cys56, Cys77, Cys75 | Cys85A, Cys55A, Cys76A, Cys74A | Attached to [Fe4S4]3+/2+ cluster. | metal ligand |
Chemical Components
electron transfer, cofactor used, coordination to a metal ion, substitution (not covered by the Ingold mechanisms), decoordination from a metal ion, native state of cofactor regenerated, proton transfer, enzyme-substrate complex formation, intermediate formation, bimolecular nucleophilic substitution, intermediate terminated, enzyme-substrate complex cleavage, native state of enzyme regenerated, overall product formedReferences
- Walters EM et al. (2005), J Am Chem Soc, 127, 9612-9624. Spectroscopic characterization of site-specific [Fe(4)S(4)] cluster chemistry in ferredoxin:thioredoxin reductase: implications for the catalytic mechanism. DOI:10.1021/ja051909q. PMID:15984889.
- Walters EM et al. (2009), Biochemistry, 48, 1016-1024. Role of histidine-86 in the catalytic mechanism of ferredoxin:thioredoxin reductase. DOI:10.1021/bi802074p. PMID:19132843.

Step 1. The interaction between the cluster and Cys87 promotes charge build-up at the unique Fe site in the resting enzyme, priming the active site for one-electron reduction from ferredoxin leading to cleavage of the disulphide and attachment of Cys87 to the cluster. Overall one-electron oxidation of the cluster to [Fe4S4]3+.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys74A | metal ligand |
| Cys76A | metal ligand |
| Cys55A | metal ligand |
| Cys85A | metal ligand |
| Cys87A | metal ligand |
| His86A | electrostatic stabiliser |
| Cys57A | proton acceptor |
| Cys87A | electrofuge, covalent catalysis |
Chemical Components
electron transfer, cofactor used, coordination to a metal ion, substitution (not covered by the Ingold mechanisms)Catalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys87A | covalent catalysis |
| His86A | proton donor, electrostatic stabiliser |
| Cys87A | proton acceptor |
Chemical Components
decoordination from a metal ion, cofactor used, electron transfer, native state of cofactor regenerated, proton transfer
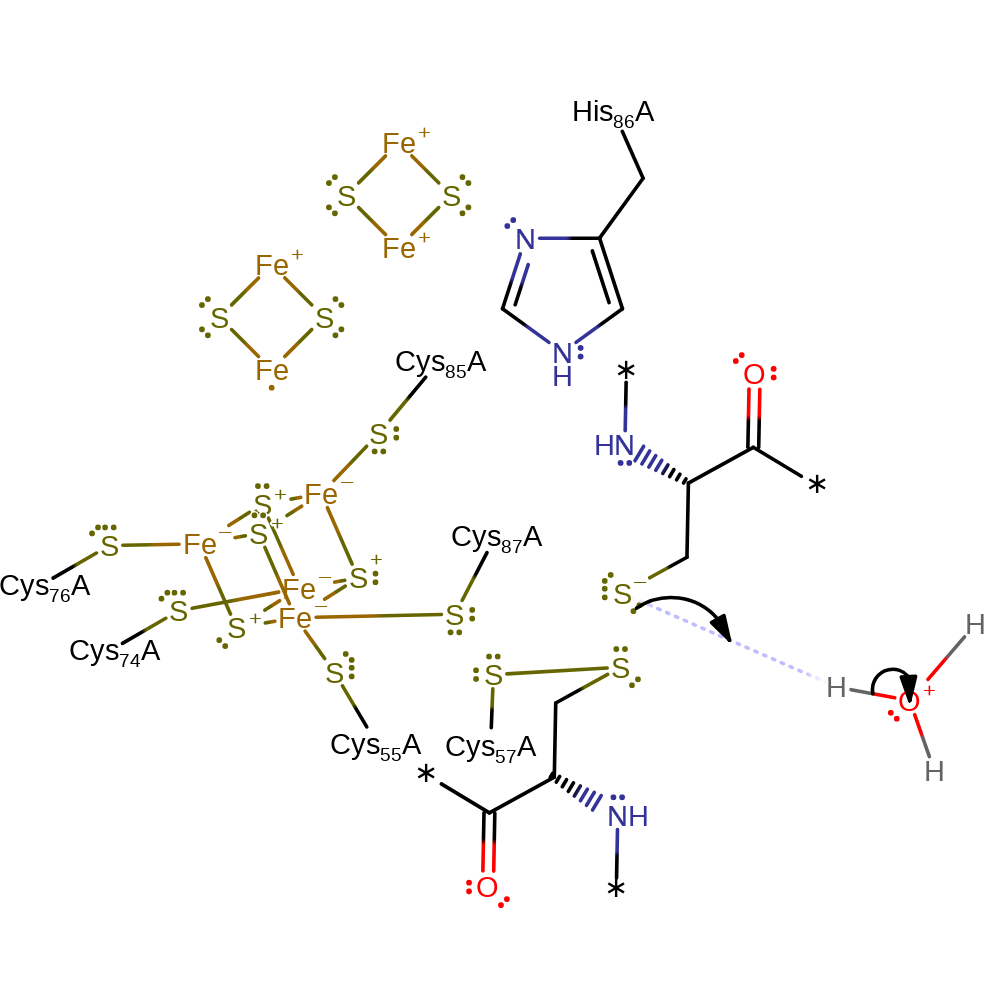
Step 3. Cys57 performs a nucleophilic attack on the thioredoxin disulphide to form an FTR/Trx heterodisulphide intermediate. This is facilitated by a hydrogen bond between Cys87 and Cys55.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys55A | hydrogen bond acceptor |
| Cys87A | hydrogen bond donor |
| His86A | electrostatic stabiliser |
| Cys85A | metal ligand |
| Cys55A | metal ligand |
| Cys76A | metal ligand |
| Cys74A | metal ligand |
| Cys57A | nucleophile, electrofuge, electrophile, proton donor |
Chemical Components
enzyme-substrate complex formation, intermediate formation, ingold: bimolecular nucleophilic substitution, proton transfer
Step 4. Cys87 reforms the active site disulphide with concomitant cleavage of the heterodisulphide intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys87A | hydrogen bond donor |
| Cys55A | hydrogen bond acceptor |
| Cys85A | metal ligand |
| Cys55A | metal ligand |
| Cys76A | metal ligand |
| Cys74A | metal ligand |
| Cys57A | nucleophile |
| Cys87A | electrophile, proton donor |
| His86A | electrostatic stabiliser |






 Download:
Download: 
 Download:
Download: