Nitrite dismutase
This enzyme is one of the nitrophorins originating from the blood feeding insect Rhodnius prolixus. Heme protein nitrophorins can catalyse the conversion of nitrite to nitric oxide (NO) with concomitant nitrate production at neutral pH. NO induces vasodilation and reduces platelet aggregation, increasing the insect's blood supply.
Reference Protein and Structure
- Sequence
-
Q94734
 (1.7.6.1)
(1.7.6.1)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Rhodnius prolixus (Triatomid bug)

- PDB
-
3mvf
- Crystal Structure of Nitrophorin 4 from Rhodnius prolixus Complexed with Nitrite at pH 7.4
(1.4 Å)



- Catalytic CATH Domains
-
2.40.128.20
 (see all for 3mvf)
(see all for 3mvf)
- Cofactors
- Heme b (1)
Enzyme Mechanism
Introduction
The mechanism for nitrite disproportionation at neutral pH uses a heme b cofactor. The heme iron is coordinated to the first substrate nitrite, which undergoes an oxygen atom transfer (OAT) to a second nitrite molecule in the protein pocket. This forms a FeNO intermediate, which reduces a third nitrite substrate yielding two NO molecules. The side-chain of Asp30 transiently stores protons required for the reaction. The exact ordering of intermediates and reactions remains ambiguous and the precise mechanism is largely inferred by the curator.
Catalytic Residues Roles
| UniProt | PDB* (3mvf) | ||
| His80 | His59A | Coordinated to heme cofactor. | metal ligand |
| Leu151 | Leu130A | L130R mutation reduces enzymatic activity. | hydrogen bond donor |
| Asp51 | Asp30A | Side-chain transiently stores protons required for the reaction. | hydrogen bond acceptor, hydrogen bond donor, proton acceptor, proton donor |
Chemical Components
intermediate formation, charge delocalisation, coordination to a metal ion, radical formation, redox reaction, proton transfer, rate-determining step, bimolecular nucleophilic addition, dehydration, electron transfer, substitution (not covered by the Ingold mechanisms)References
- He C et al. (2015), J Am Chem Soc, 137, 4141-4150. Nitrite dismutase reaction mechanism: kinetic and spectroscopic investigation of the interaction between nitrophorin and nitrite. DOI:10.1021/ja512938u. PMID:25751738.
- Di Russo NV et al. (2012), PLoS Comput Biol, 8, e1002761-. pH-Dependent conformational changes in proteins and their effect on experimental pK(a)s: the case of Nitrophorin 4. DOI:10.1371/journal.pcbi.1002761. PMID:23133364.
- Knipp M et al. (2011), IUBMB Life, 63, 304-312. Nitrophorins: nitrite disproportionation reaction and other novel functionalities of insect heme-based nitric oxide transport proteins. DOI:10.1002/iub.451. PMID:21491557.
- Benabbas A et al. (2010), J Am Chem Soc, 132, 2811-2820. Ultrafast dynamics of diatomic ligand binding to nitrophorin 4. DOI:10.1021/ja910005b. PMID:20121274.
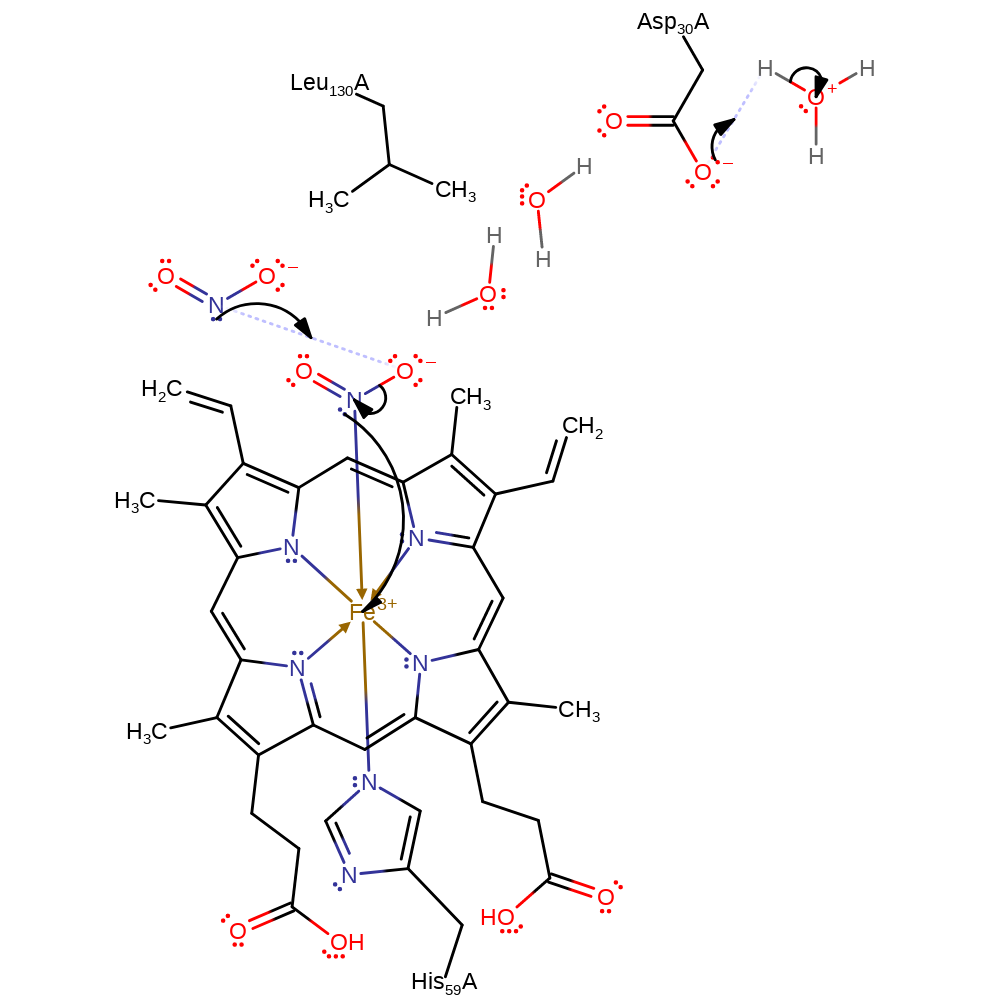
Step 1. Oxygen atom transfer (OAT) from the coordinated NO2- to a second NO2- ion in the protein pocket, forming an FeNO intermediate and an NO3- ion. Proton transfer to Asp30 from solvent.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp30A | proton acceptor |
| His59A | metal ligand |
| Asp30A | hydrogen bond acceptor |
| Leu130A | hydrogen bond donor |
Chemical Components
intermediate formation, charge delocalisation, coordination to a metal ion, radical formation, redox reaction, proton transfer, rate-determining step
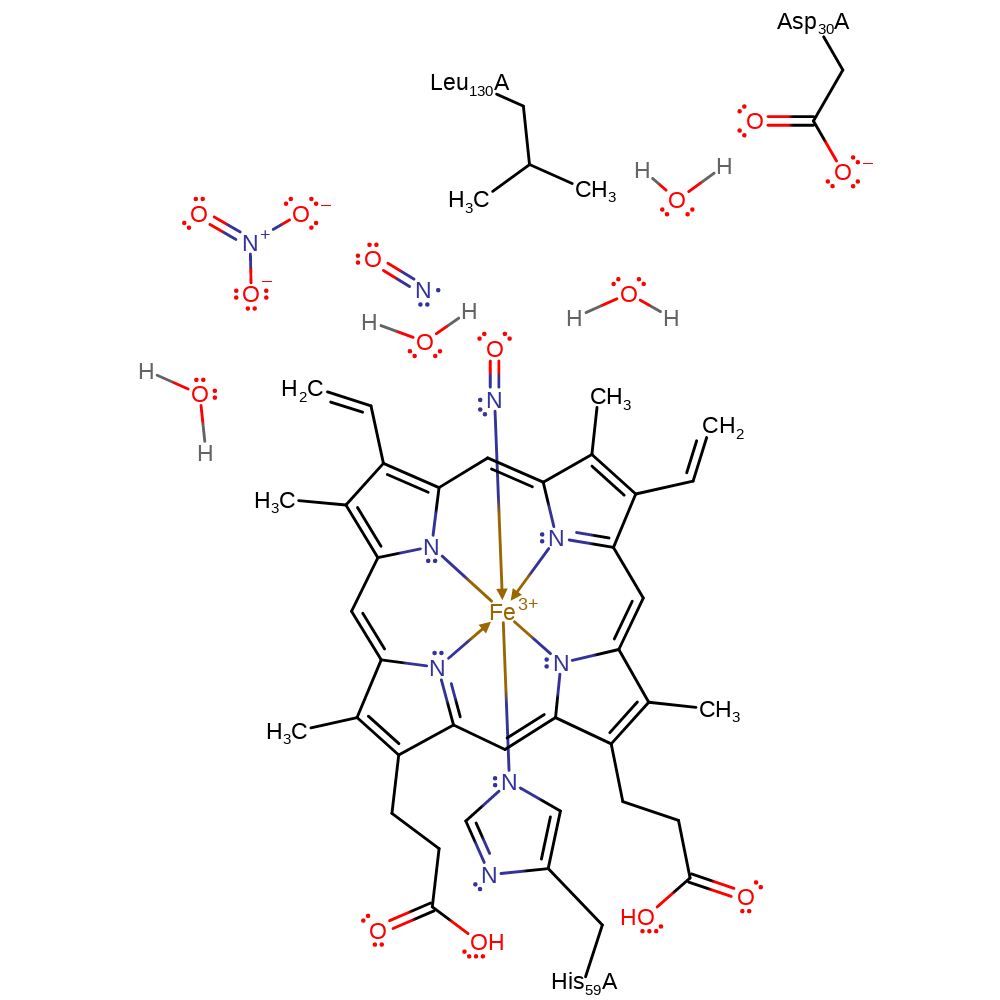
Step 2. Proton-coupled electron transfer (PCET). The FeNO intermediate reacts with a third NO2- in the protein pocket, forming a radical. Oxidation of this intermediate forms a second NO and water. The exact mechanism of this step remains uncertain and is here largely inferred by the curator.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His59A | metal ligand |
| Asp30A | proton donor, hydrogen bond donor |
Chemical Components
ingold: bimolecular nucleophilic addition, charge delocalisation, coordination to a metal ion, dehydration, electron transfer, proton transfer, redox reaction, radical formationCatalytic Residues Roles
| Residue | Roles |
|---|





 Download:
Download: