3'(2'),5'-bisphosphate nucleotidase
Catalyses the removal of the 3'-phosphate from adenosine 5'-phosphosulfate to reform AMP. This reaction helps drive the sulfur incorporation cycle for pathways such as methionine biosynthesis. Sensitivity of this essential enzyme to sodium and other metal ions results is responsible for characterisation of this enzyme as a salt tolerance protein [PMID:10205895]. Some members of this family are active also as inositol 1-monophosphatase.
Reference Protein and Structure
- Sequence
-
P32179
 (3.1.3.7)
(3.1.3.7)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Saccharomyces cerevisiae S288c (Baker's yeast)

- PDB
-
1qgx
- X-RAY STRUCTURE OF YEAST HAL2P
(1.6 Å)



- Catalytic CATH Domains
-
3.30.540.10
 3.40.190.80
3.40.190.80  (see all for 1qgx)
(see all for 1qgx)
- Cofactors
- Water (1), Magnesium(2+) (2)
Enzyme Reaction (EC:3.1.3.7)
Enzyme Mechanism
Introduction
A water molecule bound to the first site magnesium ion is activated through a proton relay with Asp49 and Thr147. It then attacks the 3'-phosphate group, eliminating the product phosphate and a negatively charged intermediate which subsequently is reprotonated from bulk solvent via a proton relay involving the second ribose hydroxyl group and a water bound to the second magnesium site.
Catalytic Residues Roles
| UniProt | PDB* (1qgx) | ||
| Asp142, Ile144 (main-C) | Asp142A, Ile144A (main-C) | Forms part of the magnesium 1 binding site. | metal ligand |
| Glu72 | Glu72A | Acts as a bridging ligand between the two magnesium ions. | metal ligand |
| Asp49, Thr147 | Asp49A, Thr147A | Asp49 and Thr147 form a proton relay chain with the catalytic water, both act as general acid/bases. Asp49 abstracts a proton from Thr147, which in turn abstracts a proton from the catalytic water, activating it for nucleophilic attack on the substrate 3'-phosphate. | proton acceptor, proton donor |
| Asp145, Asp294 | Asp145A, Asp294A | Forms part of the magnesium 2 binding site. | metal ligand |
Chemical Components
proton transfer, bimolecular nucleophilic addition, overall product formed, overall reactant used, proton relay, inferred reaction step, native state of enzyme regeneratedReferences
- Patel S et al. (2002), J Mol Biol, 320, 1087-1094. Structural Enzymology of Li+-sensitive/Mg2+-dependent Phosphatases. DOI:10.1016/s0022-2836(02)00564-8. PMID:12126627.
- Gill R et al. (2005), Acta Crystallogr D Biol Crystallogr, 61, 545-555. High-resolution structure of myo-inositol monophosphatase, the putative target of lithium therapy. DOI:10.1107/S0907444905004038. PMID:15858264.
- Albert A et al. (2000), J Mol Biol, 295, 927-938. X-ray structure of yeast Hal2p, a major target of lithium and sodium toxicity, and identification of framework interactions determining cation sensitivity. DOI:10.1006/jmbi.1999.3408. PMID:10656801.
- Gil-Mascarell R et al. (1999), Plant J, 17, 373-383. The Arabidopsis HAL2-like gene family includes a novel sodium-sensitive phosphatase. PMID:10205895.
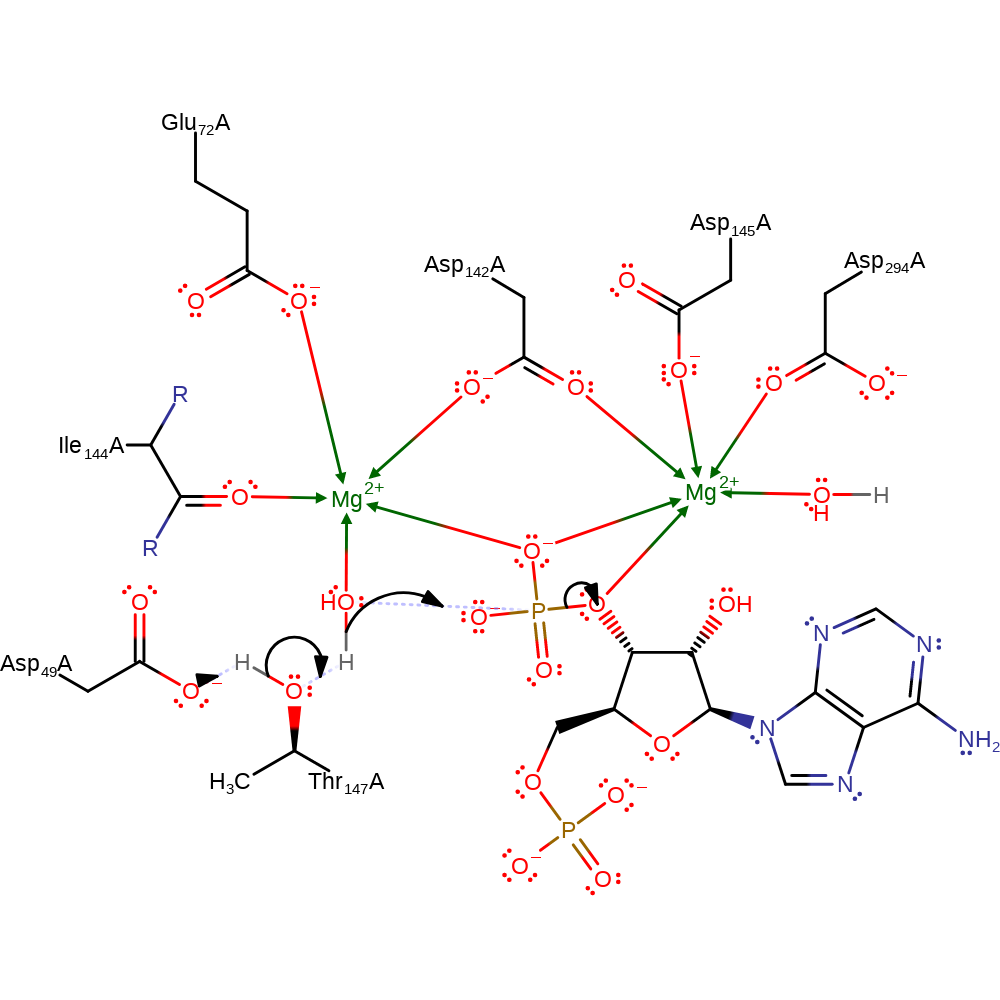
Step 1. Asp49 activates the catalytic water through Thr147. The activated species then initiates a nucleophilic attack on the magnesium bound phosphate, eliminating it from the diphosphate substrate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp294A | metal ligand |
| Asp142A | metal ligand |
| Ile144A (main-C) | metal ligand |
| Glu72A | metal ligand |
| Asp145A | metal ligand |
| Thr147A | proton acceptor |
| Asp49A | proton acceptor |
| Thr147A | proton donor, proton relay |
Chemical Components
proton transfer, ingold: bimolecular nucleophilic addition, overall product formed, overall reactant used, proton relay
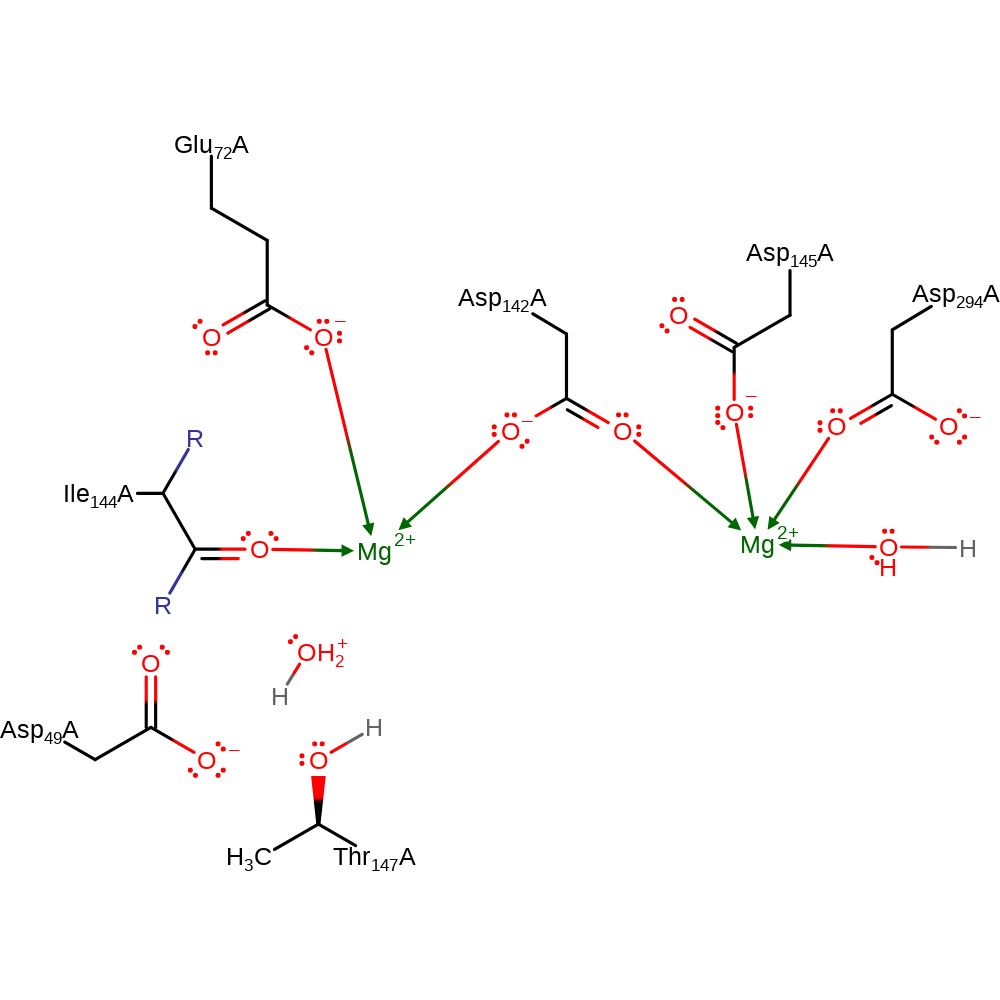
Step 2. The intermediate is reprotonated from the bulk solvent via a proton relay through the other ribose sugar, the second magnesium bound water and thence bulk solvent.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp294A | metal ligand |
| Asp142A | metal ligand |
| Ile144A (main-C) | metal ligand |
| Glu72A | metal ligand |
| Asp145A | metal ligand |
Chemical Components
overall product formed, proton relay, proton transferCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp294A | metal ligand |
| Asp142A | metal ligand |
| Ile144A (main-C) | metal ligand |
| Glu72A | metal ligand |
| Asp145A | metal ligand |
| Asp49A | proton donor |





 Download:
Download: