Caspase-9
Caspase-9 from Homo sapiens is a cysteine dependent aspartate specific protease. When activated, it will cleave the Caspase-3 peptide bond between Asp 175 and Ser 176. On existence as a monomer, Caspase-9 is inactive, but on dimerizing which is a result of the release of cytochrome c from the mitochondria cleaving procaspase-9 it becomes active, or it can be activated by making a complex with APAF1. It is involved in the process of apoptosis (programmed cell death) which is a central role in the development and homeostasis of an organism and Caspase-9 is linked to the normal development of the central nervous system.
Reference Protein and Structure
- Sequences
-
P98170
 (2.3.2.27)
(2.3.2.27)
P55211 (3.4.22.62)
(3.4.22.62)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Homo sapiens (Human)

- PDB
-
1nw9
- STRUCTURE OF CASPASE-9 IN AN INHIBITORY COMPLEX WITH XIAP-BIR3
(2.4 Å)



- Catalytic CATH Domains
-
3.40.50.1460
 (see all for 1nw9)
(see all for 1nw9)
Enzyme Reaction (EC:3.4.22.62)
Enzyme Mechanism
Introduction
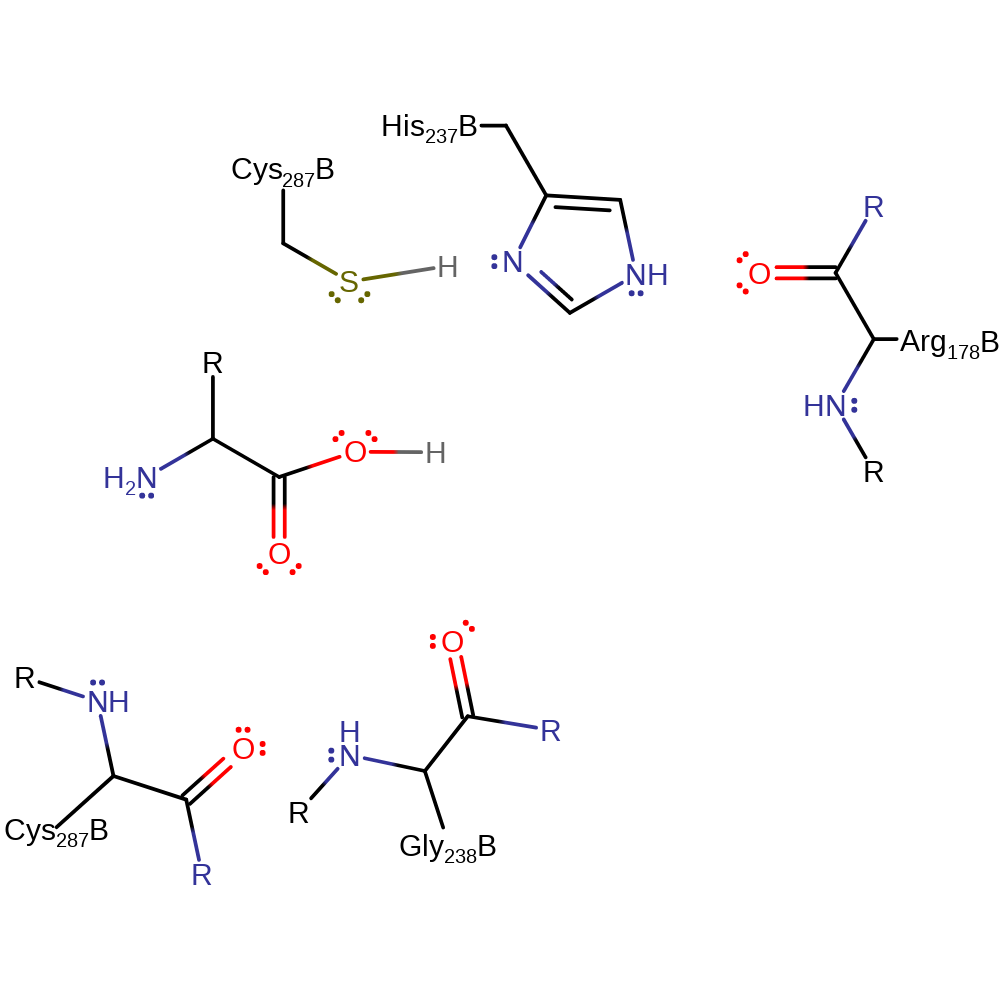
The backbone NH's of Cys 287 and Gly 238 form the oxyanion hole, and activate the scissile carbonyl bond for nucleophilic attack. His 237 is made more basic through hydrogen bonding with the oxygen atom from the backbone of Arg 178. His 237 deprotonates Cys 285 side chain, activating it so the S atom nucleophilically attacks the carbonyl, forming a negatively charged, tetrahedral intermediate. The intermediate is stabilised by hydrogen bonding to the residues forming the oxyanion hole. The carbonyl is reformed, and the leaving group amine is protonated by the previously protonated His 237. His 237 then activates a water molecule for nucleophilic attack on the carbonyl by deprotonating it. The negatively charged, tetrahedral intermediate is again stabilised by the oxyanion hole. The carbonyl is again reformed, and the protonated His 237 donates a proton to the leaving group Cys 285.
Catalytic Residues Roles
| UniProt | PDB* (1nw9) | ||
| Cys287 (main-N), Gly238 (main-N) | Cys287(148)B (main-N), Gly238(99)B (main-N) | The backbone NH of Gly 238, along with that of Cys 285, forms the oxyanion hole. Hydrogen bonds between the residue and the carbonyl oxygen of the scissile bond help to activate the carbonyl, and also to stabilise the negatively charged, tetrahedral intermediate. | electrostatic stabiliser |
| His237 | His237(98)B | His 237 deprotonates Cys 287, which then goes on to nucleophilically attack the substrate. The protonated form of His 237 then donates a proton to the leaving group amine as the carbonyl is reformed. His 237 then deprotonates a water molecule which goes on to nucleophilically attack the carbonyl. Protonated His 237 then donates a proton back to the leaving group Cys 287 as the carbonyl is once more reformed. | proton acceptor, proton donor |
| Cys287 | Cys287(148)B | The backbone NH of Cys 287 helps form the oxyanion hole by donating a H-bond to the carbonyl oxygen of the scissile bond. This makes the carbonyl more susceptible to nucleophilic attack, and also stabilises the negatively charged, tetrahedral intermediate. The side chain of Cys 287 is deprotonated by His 237, and nucleophilically attacks the carbonyl of the scissile bond, forming a negatively charged, tetrahedral intermediate. | nucleofuge, nucleophile, proton acceptor, proton donor |
| Arg178 (main-C) | Arg178(39)B (main-C) | The carbonyl oxygen of Arg 178 acts electrostatically on His 237, making it more basic, and thereby making it more ready to accept a proton from Cys 287. | electrostatic stabiliser |
Chemical Components
proton transfer, bimolecular nucleophilic addition, intermediate formation, overall reactant used, unimolecular elimination by the conjugate base, intermediate collapse, overall product formed, native state of enzyme regeneratedReferences
- Chou KC et al. (2000), FEBS Lett, 470, 249-256. Prediction of the tertiary structure of a caspase-9/inhibitor complex. DOI:10.1016/s0014-5793(00)01333-8. PMID:10745077.
- Stennicke HR et al. (1999), Cell Death Differ, 6, 1054-1059. Catalytic properties of the caspases. DOI:10.1038/sj.cdd.4400599. PMID:10578173.

Step 1. His237 deprotonates Cys287, activating it to so it can nucleophilically attack the carbon of the carbonyl which produces the oxyanion intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Gly238(99)B (main-N) | electrostatic stabiliser |
| Arg178(39)B (main-C) | electrostatic stabiliser |
| Cys287(148)B (main-N) | electrostatic stabiliser |
| His237(98)B | proton acceptor |
| Cys287(148)B | nucleophile, proton donor |
Chemical Components
proton transfer, ingold: bimolecular nucleophilic addition, intermediate formation, overall reactant used
Step 2. The oxyanion initiates an elimination which results in the cleavage of the peptide bond. His237 then donates a proton to the amino group of the N-terminal product preventing reformation of the peptide bond.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg178(39)B (main-C) | electrostatic stabiliser |
| Gly238(99)B (main-N) | electrostatic stabiliser |
| Cys287(148)B (main-N) | electrostatic stabiliser |
| His237(98)B | proton donor |
Chemical Components
ingold: unimolecular elimination by the conjugate base, proton transfer, intermediate collapse, overall product formed
Step 3. His237 deprotonates a water, activating it so that it can attack the carbon of the thioester bond in a nucleophilic addition.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg178(39)B (main-C) | electrostatic stabiliser |
| Gly238(99)B (main-N) | electrostatic stabiliser |
| Cys287(148)B (main-N) | electrostatic stabiliser |
| His237(98)B | proton acceptor |
Chemical Components
proton transfer, ingold: bimolecular nucleophilic addition, intermediate formation, overall reactant used
Step 4. The oxyanion initiates an elimination which results in the cleavage of the thioester bond. Cys287 now accepts a proton from His237 which returns the enzyme to its native state.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg178(39)B (main-C) | electrostatic stabiliser |
| Gly238(99)B (main-N) | electrostatic stabiliser |
| Cys287(148)B (main-N) | electrostatic stabiliser |
| His237(98)B | proton donor |
| Cys287(148)B | proton acceptor, nucleofuge |



 Download:
Download: