Precorrin-8X methylmutase
Precorrin-8x methyl mutase (CobH) catalyses the migration of a methyl group attached to C-11 of the substrate, precorrin-8x, to the adjacent C-12 to give the product hydrogenobyrinic acid (HBA).This is a step in the aerobic biosynthesis of the corrin macrocycle of vitamin B12.
Reference Protein and Structure
- Sequence
-
P21638
 (5.4.99.61)
(5.4.99.61)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Sinorhizobium sp.

- PDB
-
1f2v
- CRYSTAL STRUCTURE ANALYSIS OF PRECORRIN-8X METHYLMUTASE OF AEROBIC VITAMIN B12 SYNTHESIS
(2.1 Å)



- Catalytic CATH Domains
-
3.40.50.10230
 (see all for 1f2v)
(see all for 1f2v)
Enzyme Reaction (EC:5.4.99.61)
Enzyme Mechanism
Introduction
CobH is one of a few known enzymes that catalyse pericyclic reactions. His 43 protonates the nitrogen of the pyrrolenine C ring of precorrin-8x, which triggers a suprafacial [1,5]-sigmatropic rearrangement; the methyl group passes from C-11 to C-12 in a single concerted step, with the double bonds in the ring shifting position. Return of the proton to His 43 is inferred. Ser 17 is hydrogen bonded to His 43 and probably has a role in tuning the pKa of His 43, but this role is not yet explored in the literature.
Catalytic Residues Roles
| UniProt | PDB* (1f2v) | ||
| His43 | His43(52)A | His 43 acts as a proton donor to the nitrogen of the C ring of the substrate. It probably also deprotonates the product at the same position. | hydrogen bond acceptor, hydrogen bond donor, proton acceptor, proton donor |
Chemical Components
proton transfer, overall reactant used, intermediate formation, pericyclic reaction, sigmatropic rearrangement, intermediate terminated, overall product formed, native state of enzyme regeneratedReferences
- Shipman LW et al. (2001), Structure, 9, 587-596. Crystal Structure of Precorrin-8x Methyl Mutase. DOI:10.1016/s0969-2126(01)00618-9. PMID:11470433.
- Cuff ME et al. (2005), Proteins, 58, 751-754. Crystal structure of a predicted precorrin-8x methylmutase from Thermoplasma acidophilum. DOI:10.1002/prot.20022. PMID:15609338.
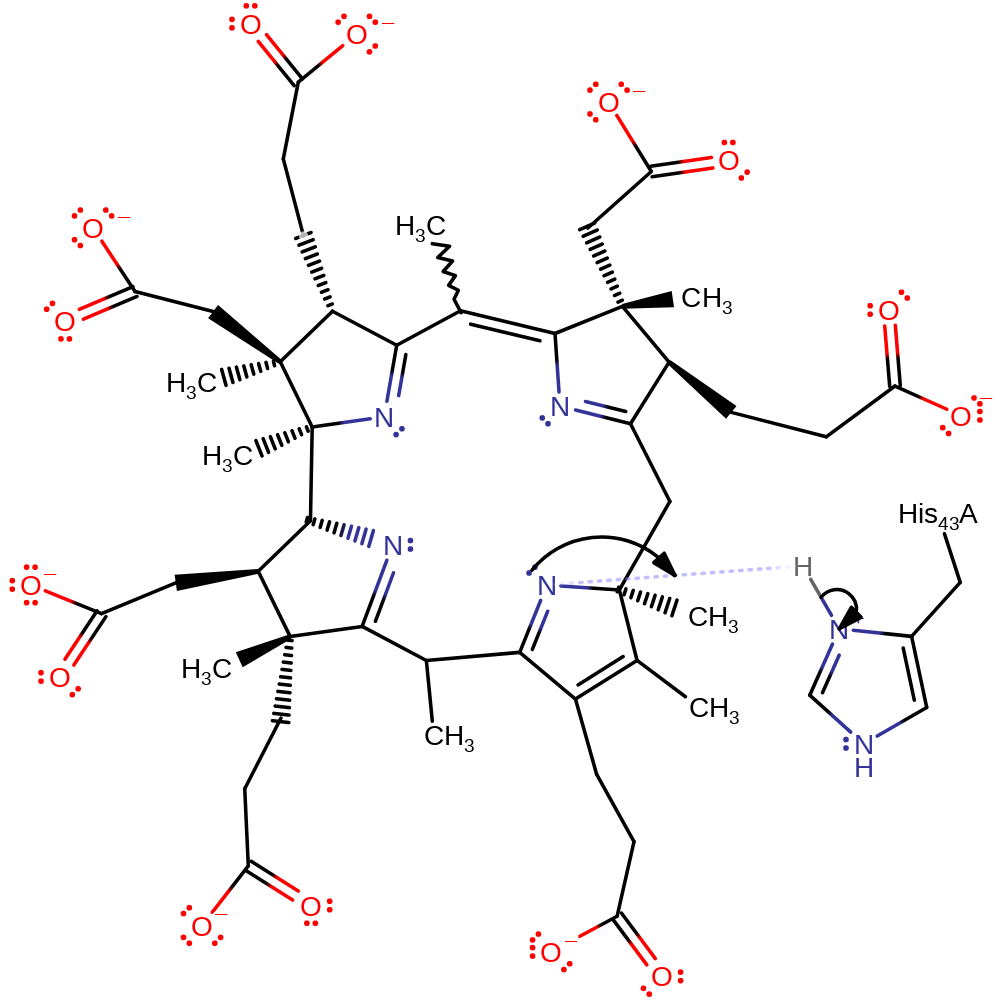
Step 1. The nitrogen of one of the precorrin 8X rings deprotonates His43.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His43(52)A | hydrogen bond donor |
| His43(52)A | proton donor |
Chemical Components
proton transfer, overall reactant used, intermediate formation
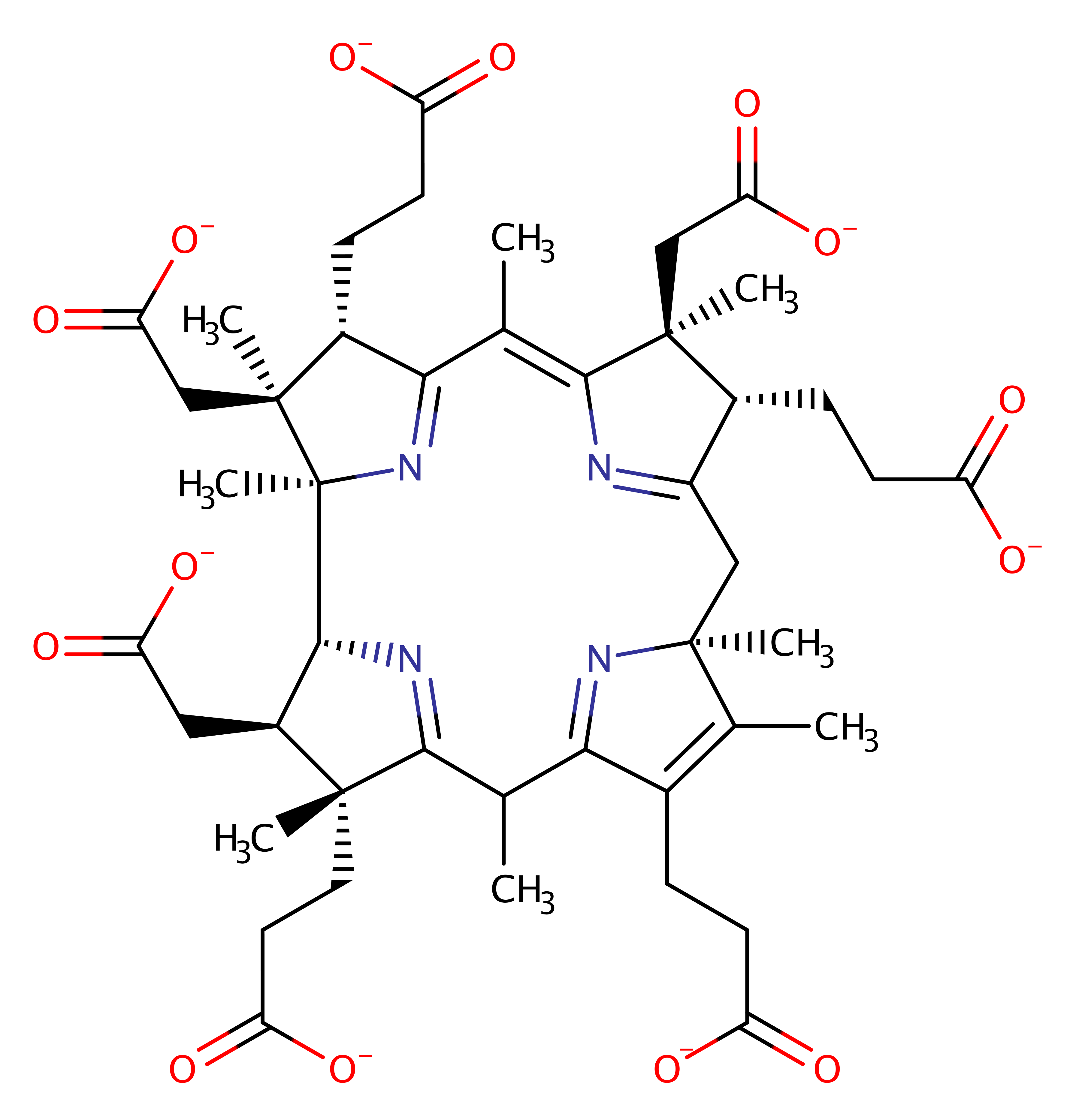
Step 2. The protonated ring then undergoes a sigmatropic rearrangement.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His43(52)A | hydrogen bond acceptor |
Chemical Components
pericyclic reaction, sigmatropic rearrangement, intermediate formation
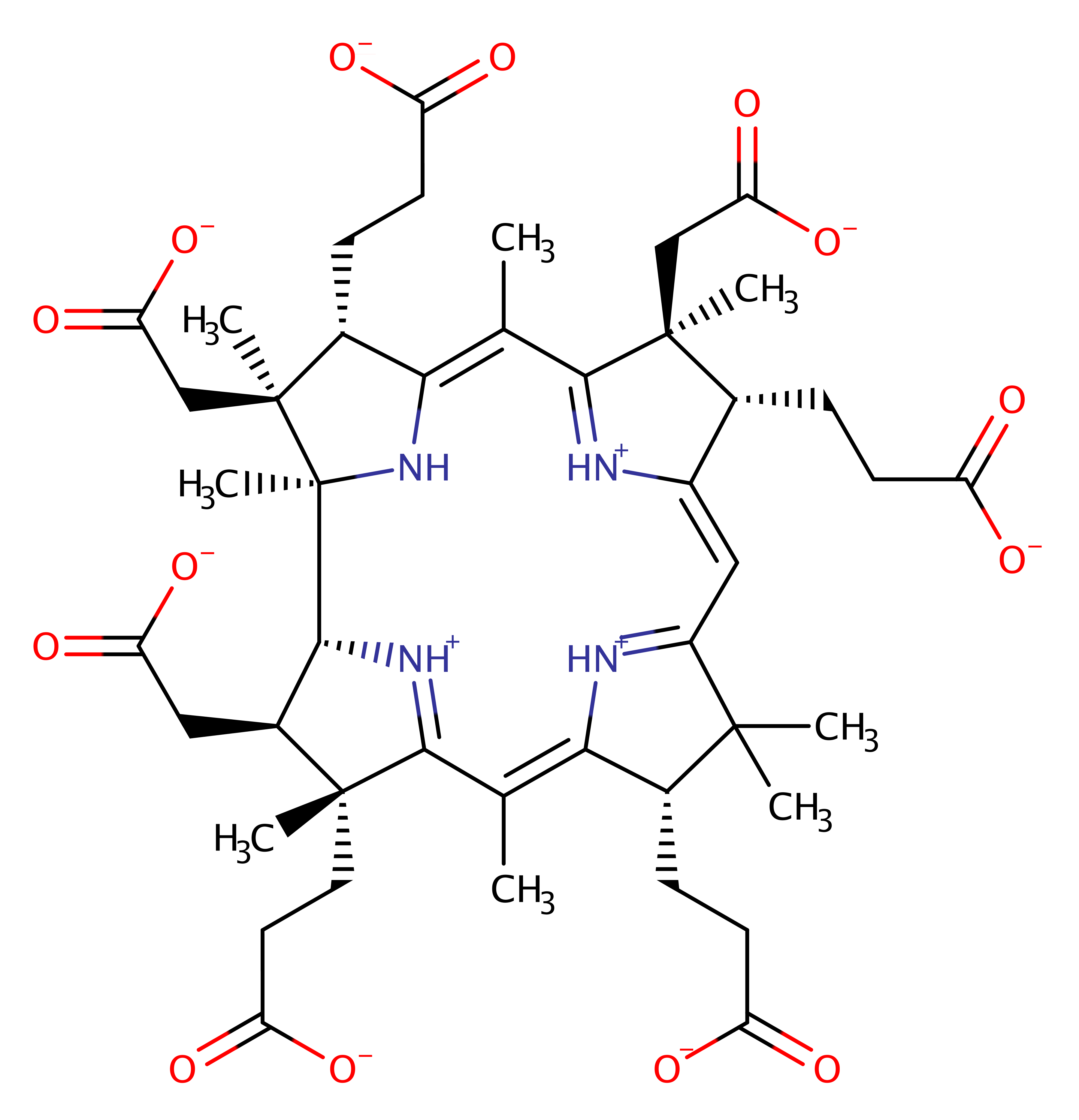
Step 3. His43 deprotonates the ring, resulting in the hydrogenobyrinate product.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His43(52)A | hydrogen bond acceptor |
| His43(52)A | proton acceptor |
 Download:
Download: