Estrone sulfotransferase
Estrogen sulfotransferase (EST) transfers the sulfate group from 3'-phosphoadenosine 5'-phosphosulfate (PAPS) to the phenolic oxygen of estrogenic steroids. EST plays a role in many carcinogenic processes, with polymorphism implicated in genetic risk towards some cancers.
Reference Protein and Structure
- Sequence
-
P49888
 (2.8.2.4)
(2.8.2.4)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Homo sapiens (Human)

- PDB
-
1hy3
- CRYSTAL STRUCTURE OF HUMAN ESTROGEN SULFOTRANSFERASE V269E MUTANT IN THE PRESENCE OF PAPS
(1.8 Å)



- Catalytic CATH Domains
-
3.40.50.300
 (see all for 1hy3)
(see all for 1hy3)
Enzyme Reaction (EC:2.8.2.4)
Enzyme Mechanism
Introduction
The mechanism is an SN2-like in-line displacement, with the phenolic hydroxy group acting as the nucleophile (made more nucleophilic by hydrogen bonding with His 107, which also transfers the phenolic proton to the sulfate group).
Lys 47 hydrogen bonds to the oxygen atom bridging PAPS 5' phosphate and sulfate groups. This promotes dissociation of the sulfate group from PAPS and may transfer a proton to the PAP product. The invariant Ser 137 plays an important regulatory role. It hydrogen bonds to Lys 47 in the absence of the steroid substrate, preventing Lys 47 assisting in hydrolysis of PAPS. Once the steroid binds, the hydrogen bond is broken and the transfer reaction can take place.
Catalytic Residues Roles
| UniProt | PDB* (1hy3) | ||
| Lys47 | Lys47A | This residue promotes dissociation of the sulfate group from PAPS and the transfer to the steroid. It also stabilises the transition state and may also donate a proton to the PAP product | hydrogen bond donor, electrostatic stabiliser |
| His107 | His107A | This residue activates the phenolic hydroxy group of the steroid substrate as a nucleophile. It also transfers the hydrogen bonded proton from the phenolic oxygen to the sulfate group. | proton relay, hydrogen bond acceptor, proton acceptor, proton donor |
| Ser137 | Ser137A | Plays an important regulatory role. Hydrogen bonds to Lys 47 in the absence of the steroid substrate, preventing Lys 47 assisting in hydrolysis of PAPS. Once the steroid binds, the hydrogen bond is broken and the transfer reaction can take place. | hydrogen bond acceptor, steric role |
Chemical Components
bimolecular nucleophilic substitution, proton transfer, overall reactant used, overall product formed, native state of enzyme regeneratedReferences
- Pedersen LC et al. (2002), J Biol Chem, 277, 17928-17932. Crystal Structure of the Human Estrogen Sulfotransferase-PAPS Complex: EVIDENCE FOR CATALYTIC ROLE OF Ser137 IN THE SULFURYL TRANSFER REACTION. DOI:10.1074/jbc.m111651200. PMID:11884392.
- Chapman E et al. (2004), Angew Chem Int Ed Engl, 43, 3526-3548. Sulfotransferases: structure, mechanism, biological activity, inhibition, and synthetic utility. DOI:10.1002/anie.200300631. PMID:15293241.
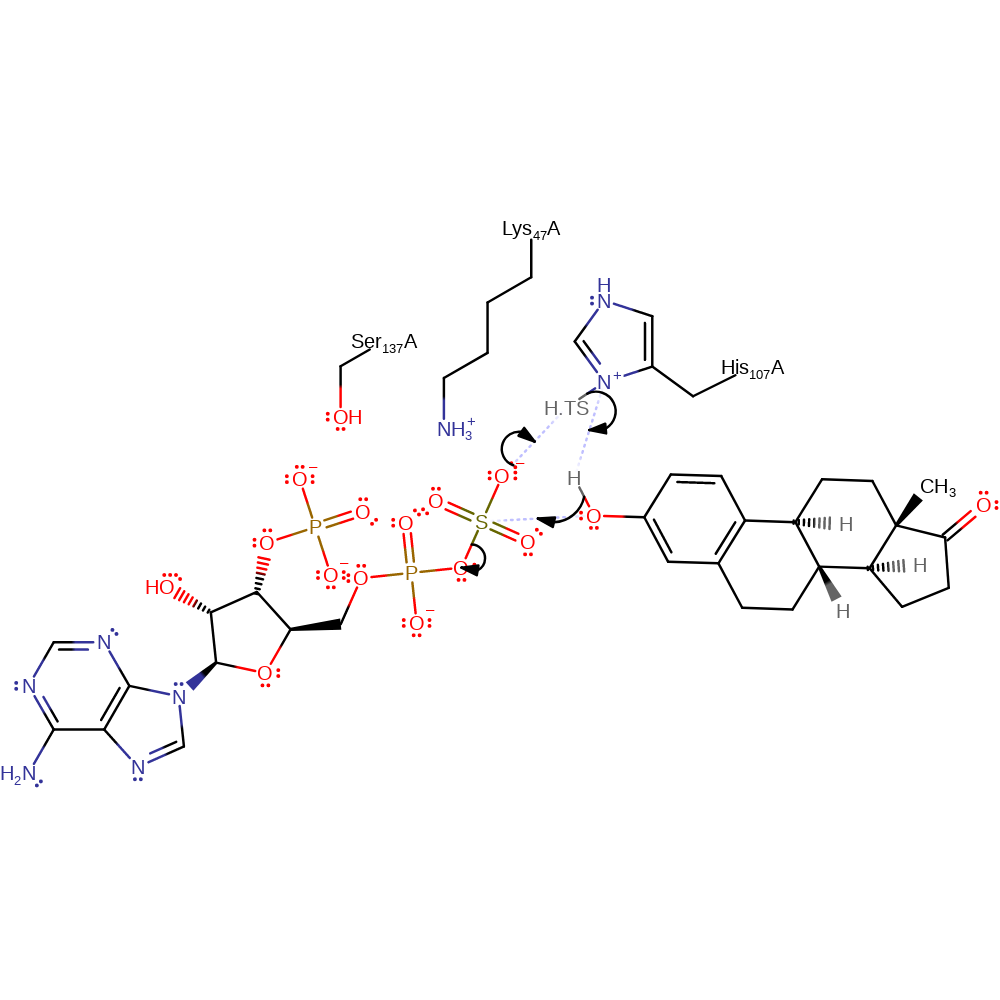
Step 1. The sulfate of 3'-phosphoadenylyl sulfate attacks the alcohol group of estrone, which then attacks the sulfate in a nucleophilic substitution. The transition state of this reaction involves a strong hydrogen bond between the His107 and estrone, which effectively results in a proton relay.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His107A | hydrogen bond acceptor, proton relay |
| Ser137A | hydrogen bond acceptor, steric role |
| Lys47A | hydrogen bond donor, electrostatic stabiliser |
| His107A | proton donor, proton acceptor |




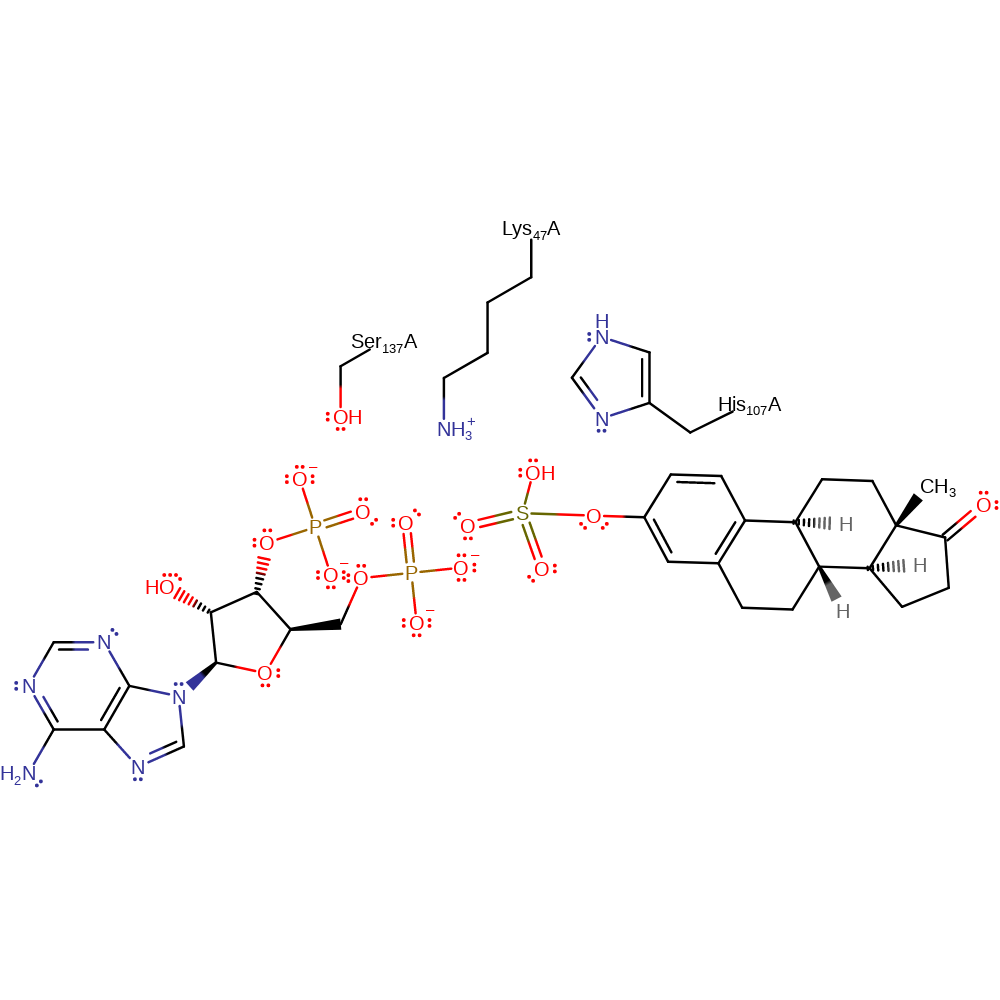 Download:
Download: