Peptidylglycine monooxygenase
peptidylglycine monooxygenase is a copper protein that acts on the terminal glycine residue of peptidylglycines. Peptidylglycines with a neutral amino acid residue in the penultimate position are the best substrates for the enzyme. In Rattus norvegicus this is functionality is part of a bifunctional enzyme. The monooxygenase part produces an unstable peptidyl(2-hydroxyglycine) as its product, which is then transported and dismutated to glyoxylate and the corresponding desglycine peptide amide by the lyase part. The C-terminal amidation of peptides such as neuropeptides is essential for full biological activity and involved in the final step of biosynthesis of alpha-melanotropin and related biologically active peptides.
Reference Protein and Structure
- Sequence
-
P14925
 (1.14.17.3, 4.3.2.5)
(1.14.17.3, 4.3.2.5)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Rattus norvegicus (Norway rat)

- PDB
-
1sdw
- Reduced (Cu+) peptidylglycine alpha-hydroxylating monooxygenase with bound peptide and dioxygen
(1.85 Å)



- Catalytic CATH Domains
-
2.60.120.230
 2.60.120.310
2.60.120.310  (see all for 1sdw)
(see all for 1sdw)
- Cofactors
- Copper(2+) (2), L-ascorbate (1), Water (3) Metal MACiE
Enzyme Reaction (EC:1.14.17.3)
Enzyme Mechanism
Introduction
Ascorbate donates a single electron to one of the Cu(II) centres, which causes the hydroxide ligand to dissociate from the Cu(II) centre. A second ascorbate donates a single electron to the other Cu(II) centre, which causes the water ligand to dissociate from the Cu(II) centre. The two semidehydroascorbates disproportionate to yield ascorbate and dehydroascorbate (it is not clear at what stage this reaction occurs. For clarity it is shown at the end of the reaction cycle as occurring outside the enzyme's active site). One of the Cu(I) centres donates a single electron to a dioxygen molecule, which causes it to bind in a bidentate manner and displace the water ligand. The bound superoxide abstracts a hydrogen from the peptide substrate, and water displaces one of the bonds between the superoxide and the Cu(II) centre. In a homolytic substitution, the O-O bond is broken resulting in the hydroxylation of the peptide intermediate and the Cu(II) bound peroxo. Water coordinates to the Cu(I) centre, initiating a single electron relay through His108, Gln170, water and the peptide product to the Cu(II) centre, which then donates the electron to the bound oxo group, which deprotonates a water molecule to regenerate the active site.
Catalytic Residues Roles
| UniProt | PDB* (1sdw) | ||
| His108 | His108(66)A | Part if the Copper A binding site (also known as the CuM site), and part of a single electon relay chain that links the two copper sites. | single electron relay, hydrogen bond donor, metal ligand, single electron acceptor, single electron donor |
| Gln170 | Gln170(128)A | Part of the single electron relay chain that links the two copper sites. | single electron relay, single electron donor, single electron acceptor, hydrogen bond acceptor |
| His107, His172 | His107(65)A, His172(130)A | Forms part of the Copper A binding site (also known as the CuM site). | metal ligand |
| His244, Met314, His242 | His244(202)A, Met314(272)A, His242(200)A | Forms part of the Copper B binding site (also known as the CuH site). | metal ligand |
Chemical Components
redox reaction, radical formation, elimination (not covered by the Ingold mechanisms), overall reactant used, decoordination from a metal ion, intermediate formation, cofactor used, bimolecular homolytic addition, bimolecular nucleophilic substitution, coordination to a metal ion, hydrogen transfer, radical propagation, bimolecular homolytic substitution, overall product formed, proton transfer, electron transfer, radical termination, coordination, intermediate terminated, native state of enzyme regenerated, electron relay, reaction occurs outside the enzyme, native state of cofactor regeneratedReferences
- Chen P et al. (2004), J Am Chem Soc, 126, 4991-5000. Oxygen Activation by the Noncoupled Binuclear Copper Site in Peptidylglycine α-Hydroxylating Monooxygenase. Reaction Mechanism and Role of the Noncoupled Nature of the Active Site. DOI:10.1021/ja031564g. PMID:15080705.
- Abad E et al. (2014), J Biol Chem, 289, 13726-13738. Reaction mechanism of the bicopper enzyme peptidylglycine α-hydroxylating monooxygenase. DOI:10.1074/jbc.M114.558494. PMID:24668808.
- Chauhan S et al. (2014), Biochemistry, 53, 1069-1080. Binding of copper and silver to single-site variants of peptidylglycine monooxygenase reveals the structure and chemistry of the individual metal centers. DOI:10.1021/bi4015264. PMID:24471980.
- Rudzka K et al. (2013), J Biol Inorg Chem, 18, 223-232. Coordination of peroxide to the Cu(M) center of peptidylglycine α-hydroxylating monooxygenase (PHM): structural and computational study. DOI:10.1007/s00775-012-0967-z. PMID:23247335.
- Meliá C et al. (2013), Chemistry, 19, 17328-17337. Investigation of the hydroxylation mechanism of noncoupled copper oxygenases by ab initio molecular dynamics simulations. DOI:10.1002/chem.201301000. PMID:24259416.
- Kline CD et al. (2013), Biochemistry, 52, 2586-2596. HHM motif at the CuH-site of peptidylglycine monooxygenase is a pH-dependent conformational switch. DOI:10.1021/bi4002248. PMID:23530865.
- Bauman AT et al. (2011), Biochemistry, 50, 10819-10828. A copper-methionine interaction controls the pH-dependent activation of peptidylglycine monooxygenase. DOI:10.1021/bi201193j. PMID:22080626.
- Chufán EE et al. (2010), J Am Chem Soc, 132, 15565-15572. Differential reactivity between two copper sites in peptidylglycine α-hydroxylating monooxygenase. DOI:10.1021/ja103117r. PMID:20958070.
- Woertink JS et al. (2010), Inorg Chem, 49, 9450-9459. Spectroscopic and computational studies of an end-on bound superoxo-Cu(II) complex: geometric and electronic factors that determine the ground state. DOI:10.1021/ic101138u. PMID:20857998.
- McIntyre NR et al. (2009), J Am Chem Soc, 131, 10308-10319. Imino-oxy acetic acid dealkylation as evidence for an inner-sphere alcohol intermediate in the reaction catalyzed by peptidylglycine alpha-hydroxylating monooxygenase. DOI:10.1021/ja902716d. PMID:19569683.
- Bauman AT et al. (2006), J Biol Chem, 281, 4190-4198. The hydrogen peroxide reactivity of peptidylglycine monooxygenase supports a Cu(II)-superoxo catalytic intermediate. DOI:10.1074/jbc.M511199200. PMID:16330540.
- Siebert X et al. (2005), Biophys J, 89, 3312-3319. The catalytic copper of peptidylglycine alpha-hydroxylating monooxygenase also plays a critical structural role. DOI:10.1529/biophysj.105.066100. PMID:16100265.
- Prigge ST et al. (2004), Science, 304, 864-867. Dioxygen Binds End-On to Mononuclear Copper in a Precatalytic Enzyme Complex. DOI:10.1126/science.1094583. PMID:15131304.
- Prigge ST et al. (2000), Cell Mol Life Sci, 57, 1236-1259. New insights into copper monooxygenases and peptide amidation: structure, mechanism and function. DOI:10.1007/pl00000763. PMID:11028916.
- Prigge ST et al. (1999), Nat Struct Biol, 6, 976-983. Substrate-mediated electron transfer in peptidylglycine alpha-hydroxylating monooxygenase. DOI:10.1038/13351. PMID:10504734.
- Prigge ST et al. (1997), Science, 278, 1300-1305. Amidation of bioactive peptides: the structure of peptidylglycine alpha-hydroxylating monooxygenase. PMID:9360928.
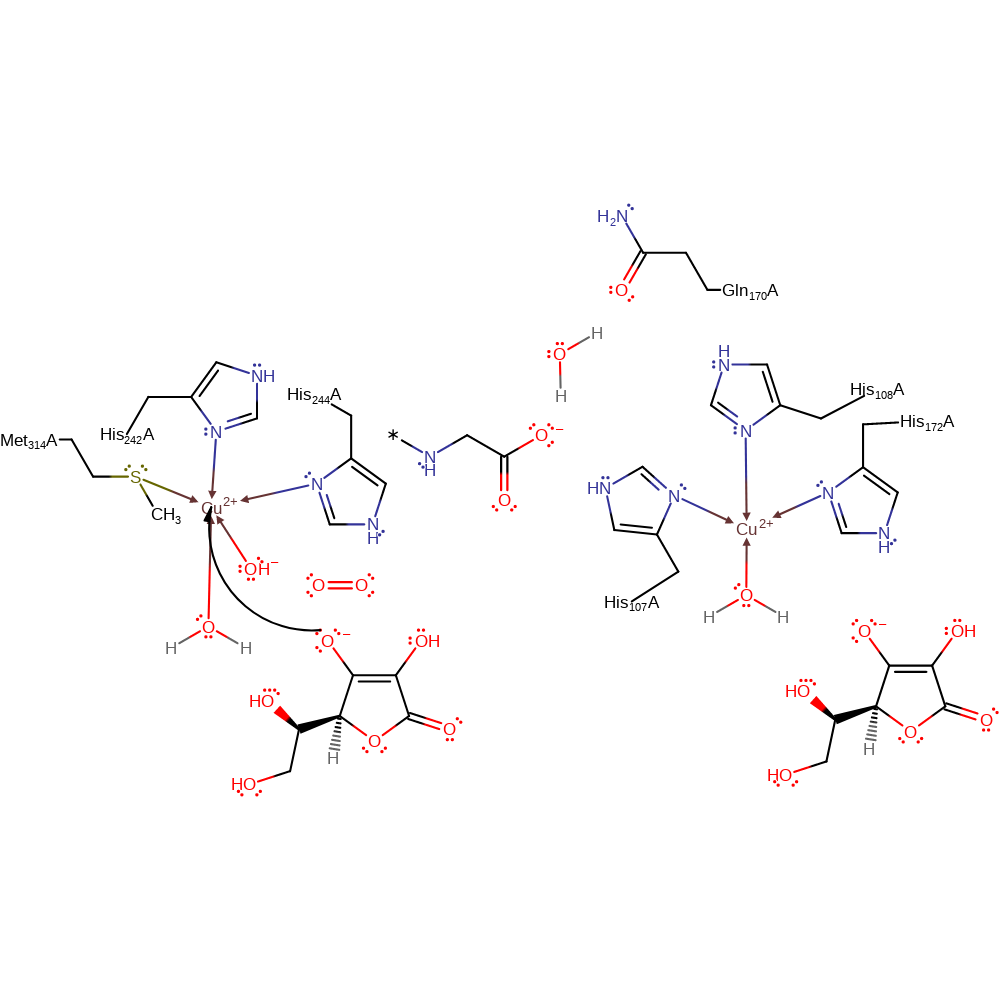
Step 1. Ascorbate donates a single electron to one of the Cu(II) centres, which causes the hydroxide ligand to dissociate from the Cu(II) centre.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His108(66)A | hydrogen bond donor |
| Gln170(128)A | hydrogen bond acceptor |
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
Chemical Components
redox reaction, radical formation, elimination (not covered by the Ingold mechanisms), overall reactant used, decoordination from a metal ion, intermediate formation, cofactor used
Step 2. A second ascorbate donates a single electron to the other Cu(II) centre, which causes the water ligand to dissociate from the Cu(II) centre.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His108(66)A | hydrogen bond donor |
| Gln170(128)A | hydrogen bond acceptor |
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
Chemical Components
redox reaction, radical formation, elimination (not covered by the Ingold mechanisms), overall reactant used, decoordination from a metal ion, intermediate formation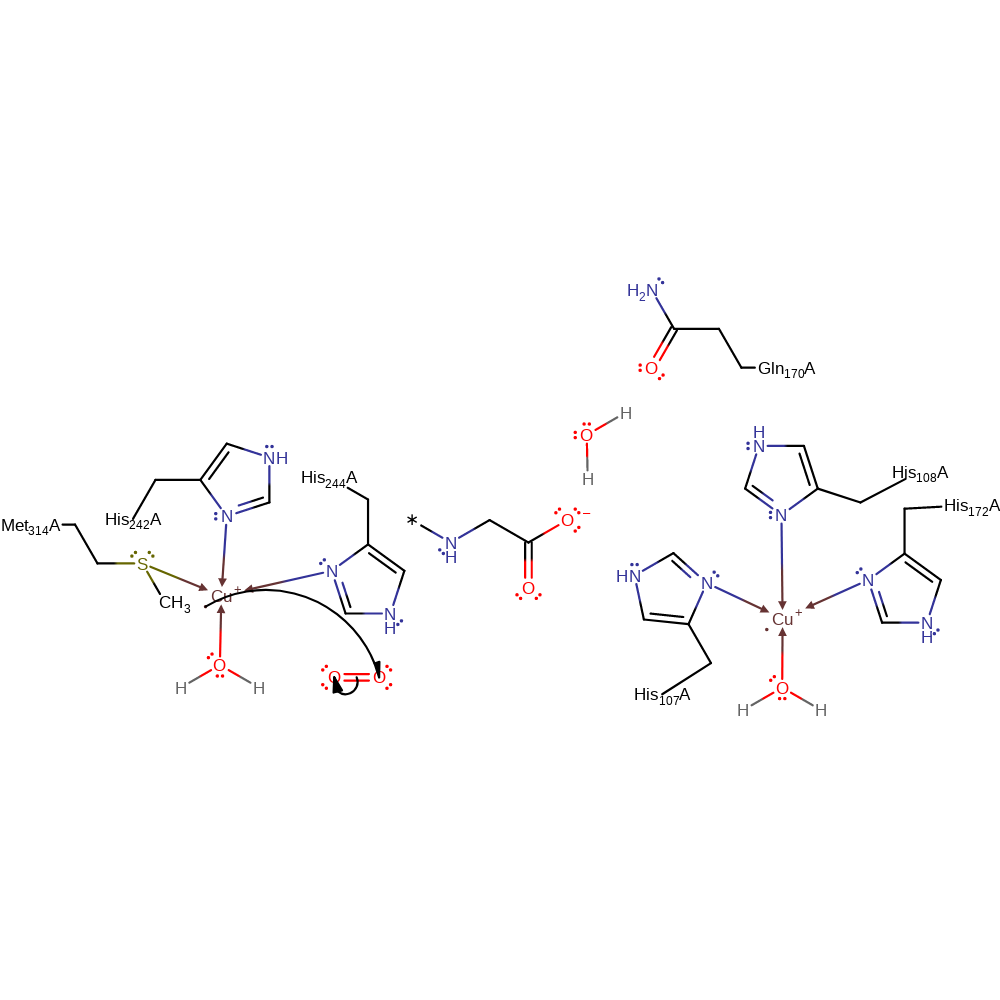
Step 3. One of the Cu(I) centres donates a single electron to a dioxygen molecule, which causes it to bind in a bidentate manner and displace the water ligand.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His108(66)A | hydrogen bond donor |
| Gln170(128)A | hydrogen bond acceptor |
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
Chemical Components
ingold: bimolecular homolytic addition, redox reaction, radical formation, ingold: bimolecular nucleophilic substitution, overall reactant used, coordination to a metal ion, decoordination from a metal ion, intermediate formation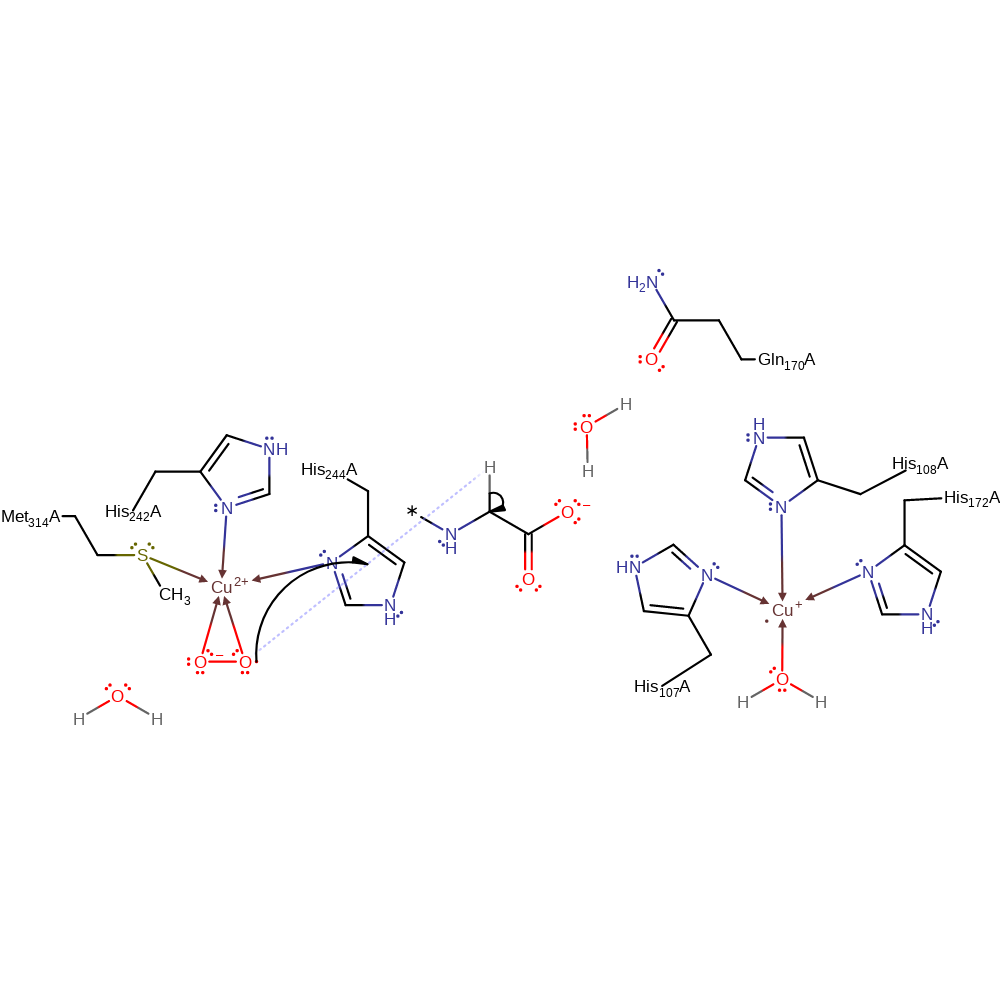
Step 4. The bound superoxide abstracts a hydrogen from the peptide substrate, and water displaces one of the bonds between the superoxide and the Cu(II) centre.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His108(66)A | hydrogen bond donor |
| Gln170(128)A | hydrogen bond acceptor |
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
Chemical Components
ingold: bimolecular nucleophilic substitution, hydrogen transfer, radical propagation, overall reactant used, coordination to a metal ion, decoordination from a metal ion, intermediate formation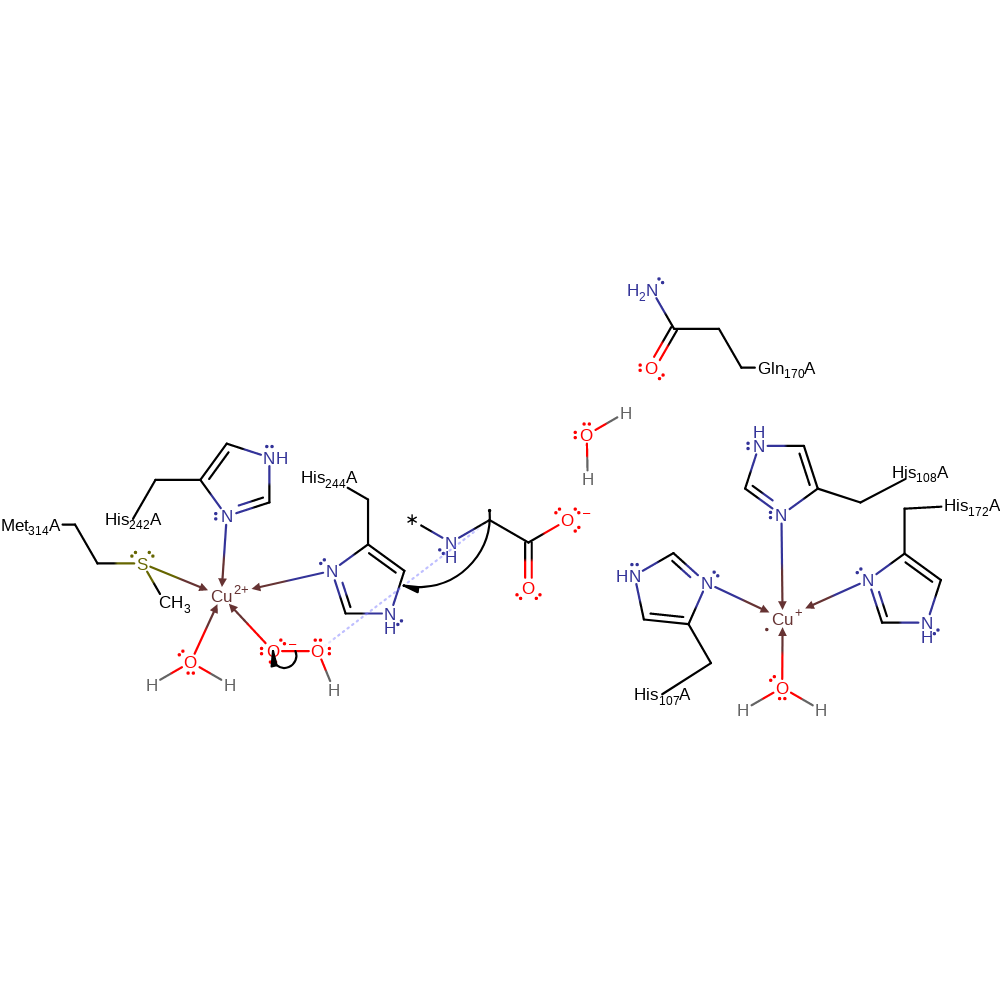
Step 5. In a homolytic substitution, the O-O bond is broken resulting in the hydroxylation of the peptide intermediate and the Cu(II) bound peroxo.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His108(66)A | hydrogen bond donor |
| Gln170(128)A | hydrogen bond acceptor |
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
Chemical Components
ingold: bimolecular homolytic substitution, radical propagation, intermediate formation, overall product formed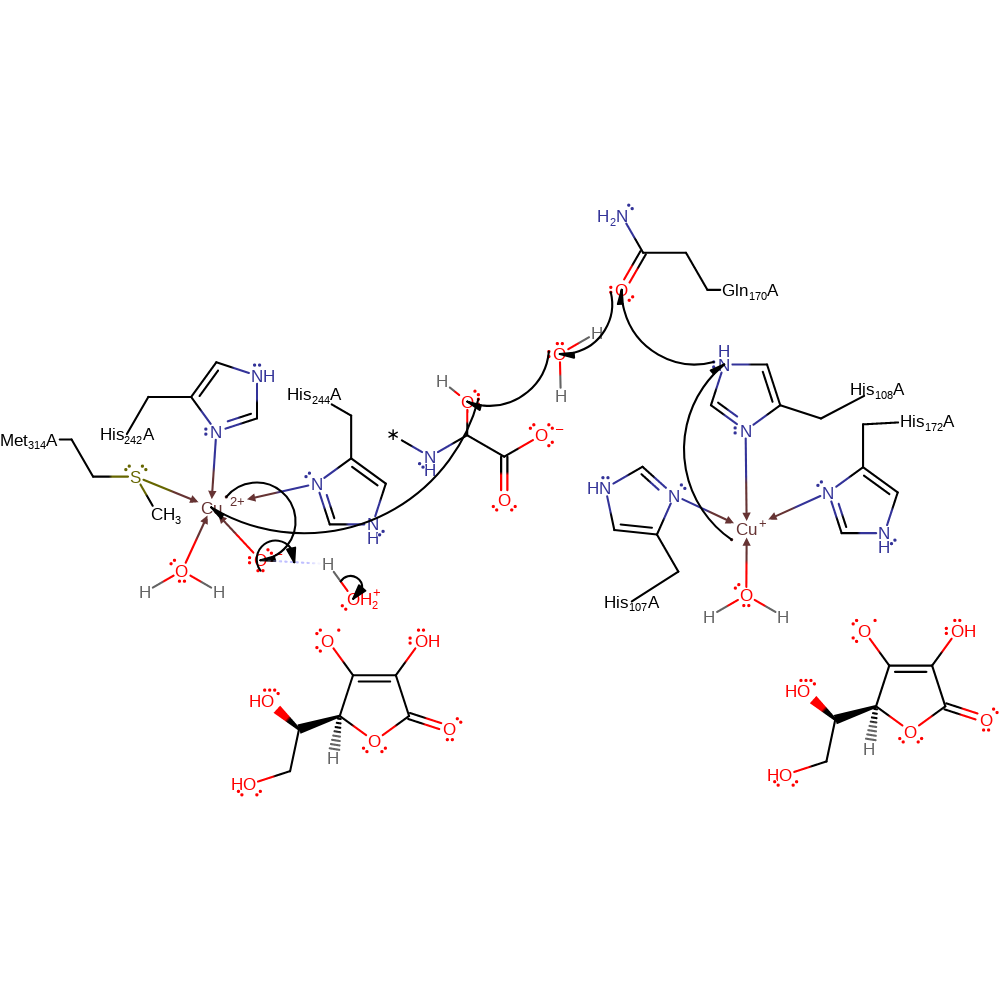
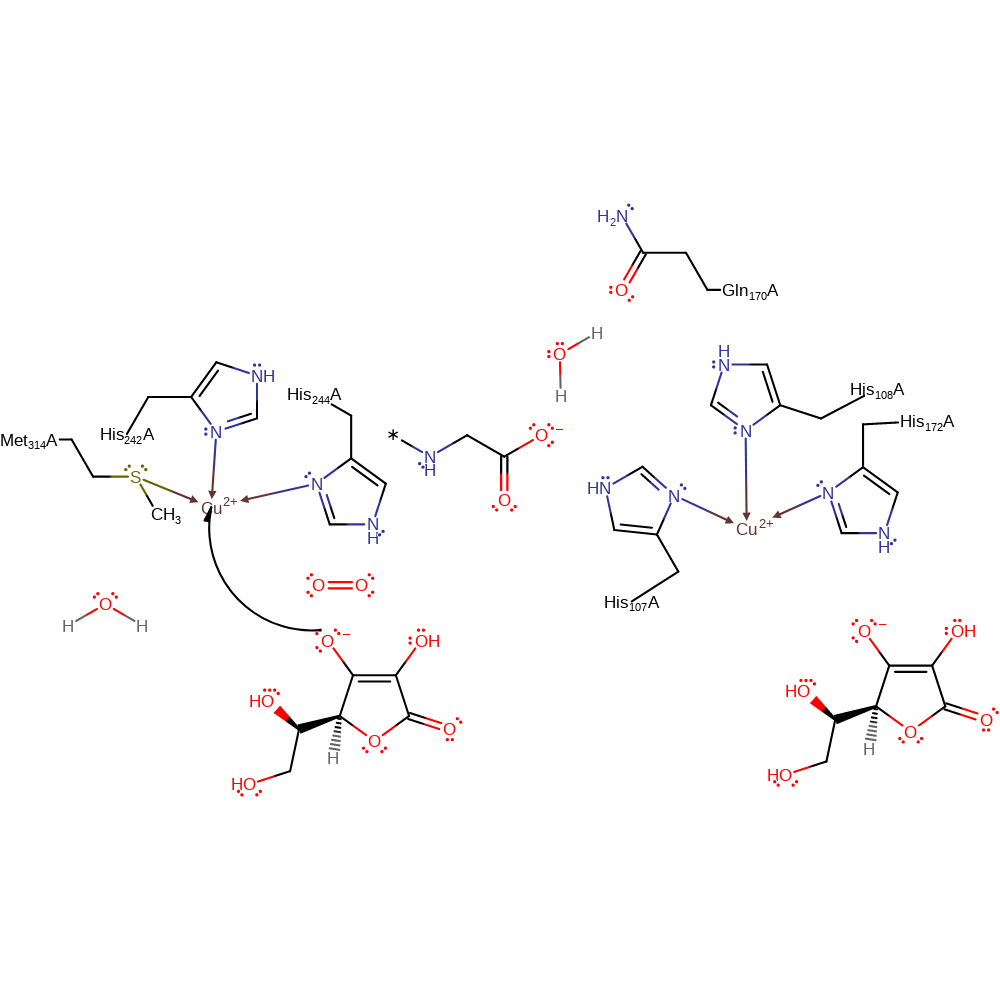
Step 6. Water coordinates to the Cu(I) centre, initiating a single electron relay through His108, Gln170, water and the peptide product to the Cu(II) centre, which then donates the electron to the bound oxo group, which deprotonates a water molecule to regenerate the active site.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His108(66)A | hydrogen bond donor |
| Gln170(128)A | hydrogen bond acceptor |
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
| His108(66)A | single electron relay |
| Gln170(128)A | single electron relay, single electron donor |
| His108(66)A | single electron acceptor, single electron donor |
| Gln170(128)A | single electron acceptor |
Chemical Components
proton transfer, electron transfer, radical termination, coordination, coordination to a metal ion, intermediate terminated, native state of enzyme regenerated, electron relay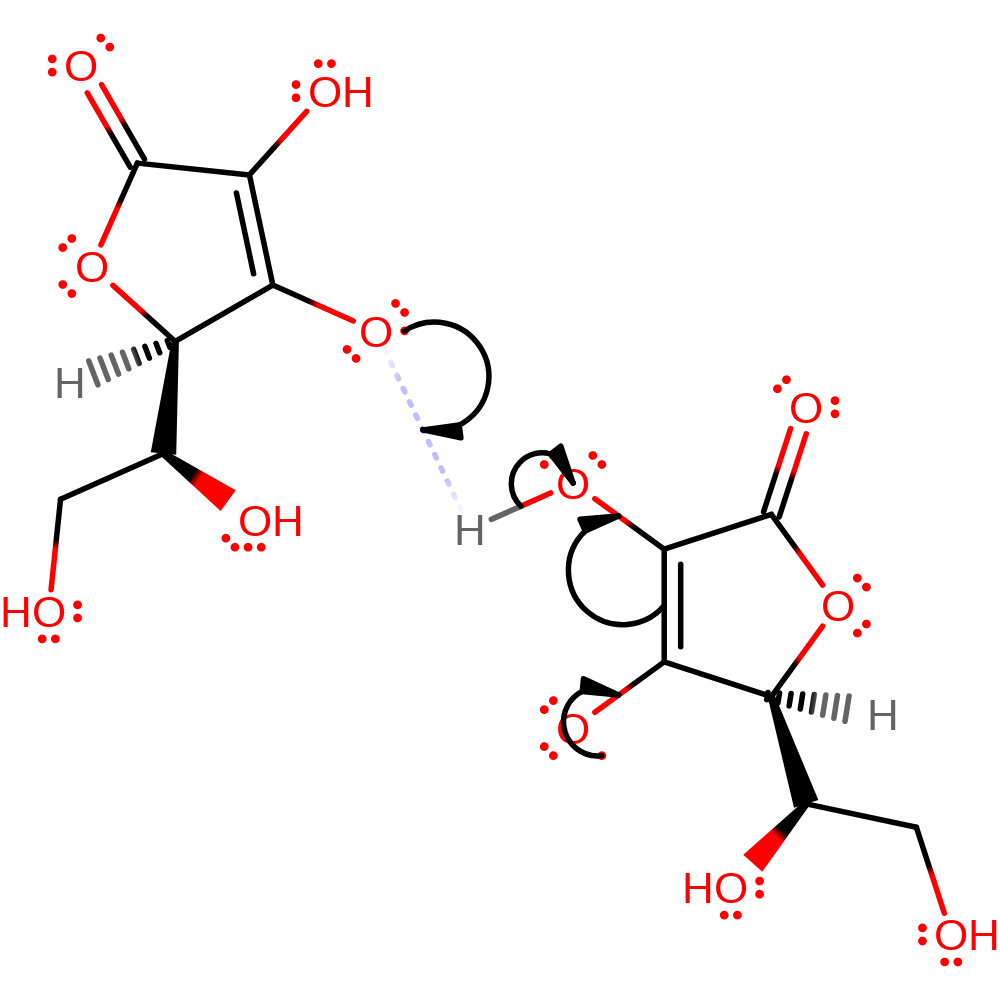
Step 7. The two semidehydroascorbates disproportionate to yield ascorbate and dehydroascorbate [PMID:11028916].
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|
Chemical Components
reaction occurs outside the enzyme, native state of cofactor regenerated, overall product formedIntroduction
The 2 copper centres will be reduced by the ascorbarte cofactors which will then result in one of the reduced copper centres donating an electron to a dioxygen molecule. The Cu(II)-superoxo intermediate abstracts a hydrogen from the substrate produces a Cu(II)-hydroperoxo species which will then follow an end-on mechanism is reduced via an intramolecular electron transfer from the other Cu atom in the active site yielding a CuII-O• radical. Radical recombination of the substrate and Cu-O radical species produces an innersphere alcohol intermediate. Subsequent hydrolysis of the innersphere alcohol generates the hydroxylated product.
Catalytic Residues Roles
| UniProt | PDB* (1sdw) | ||
| His108 | His108(66)A | Part if the Copper A binding site (also known as the CuM site), and part of a single electon relay chain that links the two copper sites. | single electron relay, hydrogen bond donor, metal ligand, single electron acceptor, single electron donor |
| Gln170 | Gln170(128)A | Part of the single electron relay chain that links the two copper sites. | single electron relay, single electron donor, single electron acceptor, hydrogen bond acceptor |
| His107, His172 | His107(65)A, His172(130)A | Forms part of the Copper A binding site (also known as the CuM site). | metal ligand |
| His244, Met314, His242 | His244(202)A, Met314(272)A, His242(200)A | Forms part of the Copper B binding site (also known as the CuH site). | metal ligand |
Chemical Components
redox reaction, radical formation, intermediate formation, cofactor used, bimolecular homolytic addition, overall reactant used, coordination to a metal ion, homolysis, bimolecular homolytic substitution, hydrogen transfer, radical propagation, proton transfer, electron relay, electron transfer, inferred reaction step, colligation, radical termination, elimination (not covered by the Ingold mechanisms), intermediate collapse, intermediate terminated, overall product formed, native state of cofactor regenerated, reaction occurs outside the enzymeReferences
- McIntyre NR et al. (2009), J Am Chem Soc, 131, 10308-10319. Imino-oxy acetic acid dealkylation as evidence for an inner-sphere alcohol intermediate in the reaction catalyzed by peptidylglycine alpha-hydroxylating monooxygenase. DOI:10.1021/ja902716d. PMID:19569683.
Step 1. Ascorbate donates a single electron to one of the Cu(II) centres, which causes the hydroxide ligand to dissociate from the Cu(II) centre.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Met314(272)A | metal ligand |
| His244(202)A | metal ligand |
| His242(200)A | metal ligand |
| His172(130)A | metal ligand |
| His108(66)A | metal ligand |
| His107(65)A | metal ligand |
| His108(66)A | hydrogen bond donor |
| Gln170(128)A | hydrogen bond acceptor |
Chemical Components
redox reaction, radical formation, intermediate formation, cofactor used
Step 2. A second ascorbate donates a single electron to the other Cu(II) centre, which will also convert the other copper centre to Cu(I).
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Met314(272)A | metal ligand |
| His244(202)A | metal ligand |
| His242(200)A | metal ligand |
| His172(130)A | metal ligand |
| His108(66)A | metal ligand |
| His107(65)A | metal ligand |
| His108(66)A | hydrogen bond donor |
| Gln170(128)A | hydrogen bond acceptor |
Chemical Components
redox reaction, radical formation, intermediate formation, cofactor used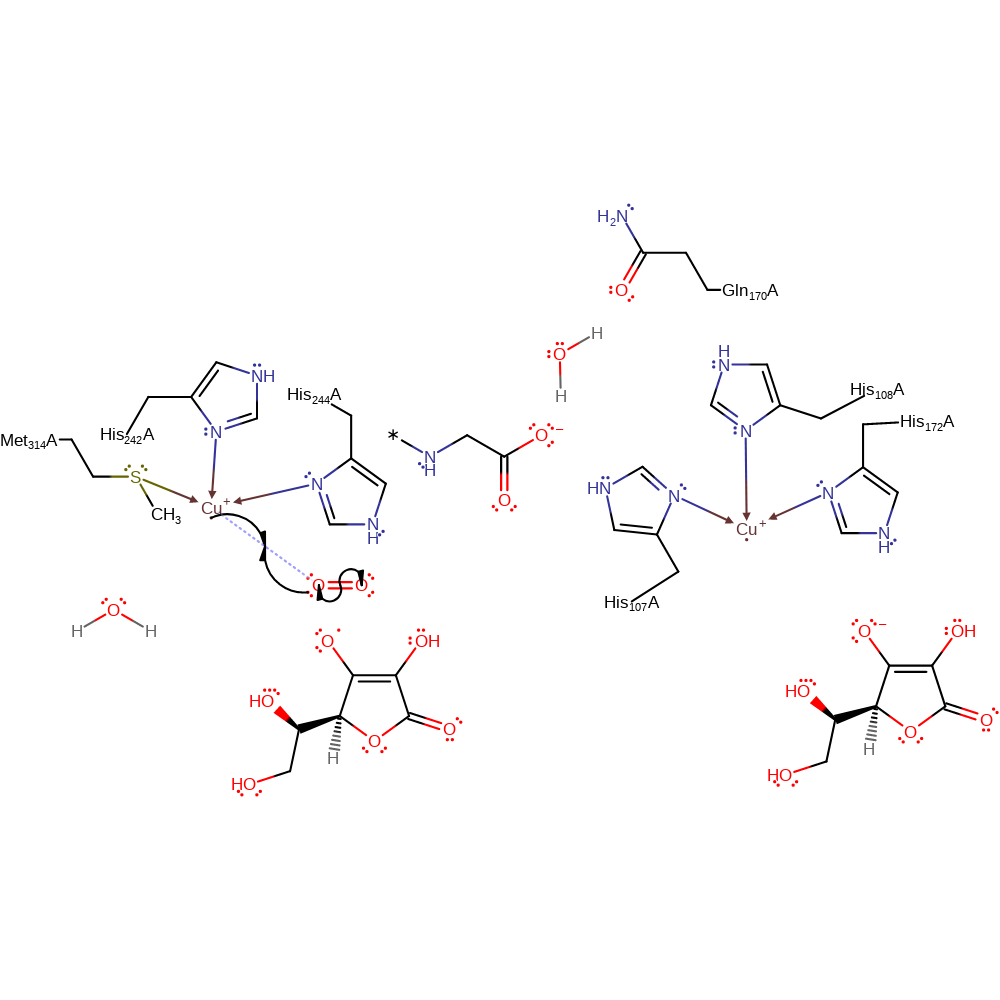
Step 3. One of the Cu(I) centres donates a single electron to a dioxygen molecule, which causes it to bind to the copper centre.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
| Gln170(128)A | hydrogen bond acceptor |
| His108(66)A | hydrogen bond donor |
Chemical Components
ingold: bimolecular homolytic addition, redox reaction, radical formation, overall reactant used, coordination to a metal ion, homolysis, intermediate formation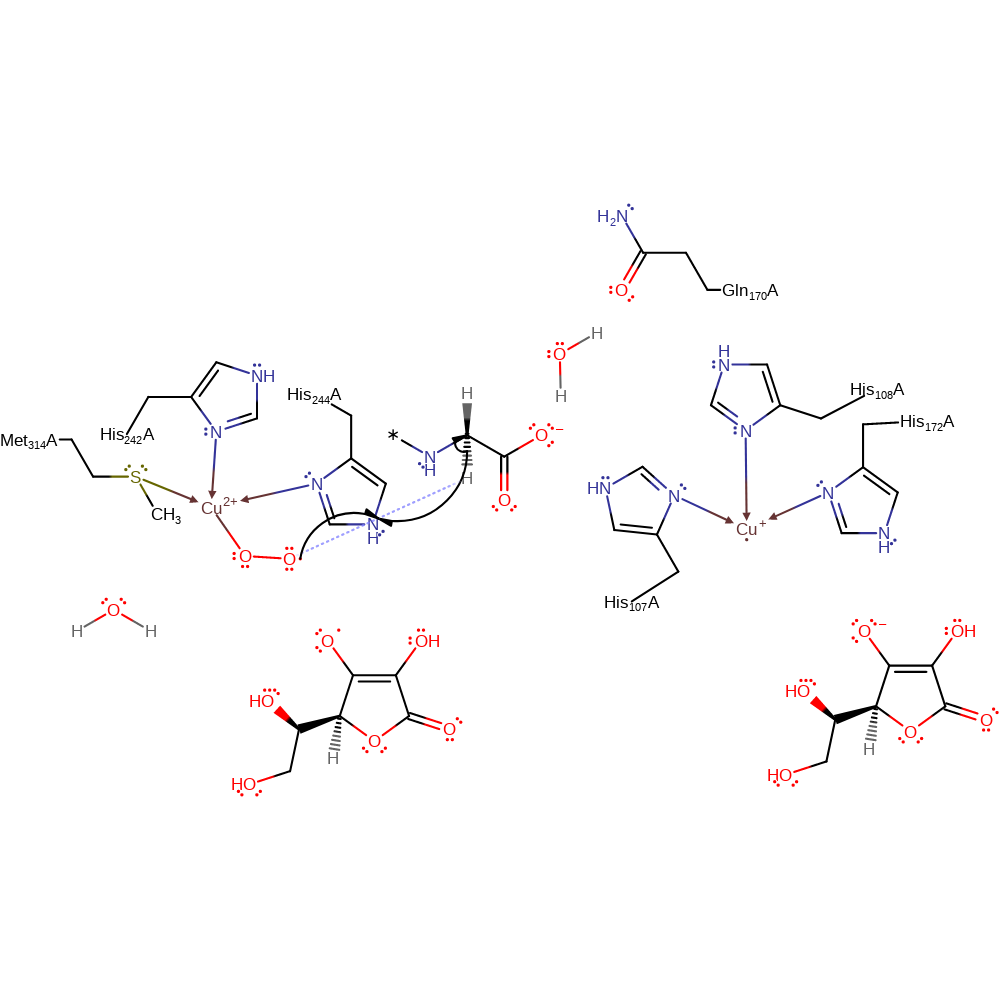
Step 4. The Cu(II)-superoxo abstracts a hydrogen from the substrate producing Cu(II)-hydroperoxo and a radical substrate species.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
| Gln170(128)A | hydrogen bond acceptor |
| His108(66)A | hydrogen bond donor |
Chemical Components
ingold: bimolecular homolytic substitution, hydrogen transfer, radical propagation, overall reactant used, intermediate formation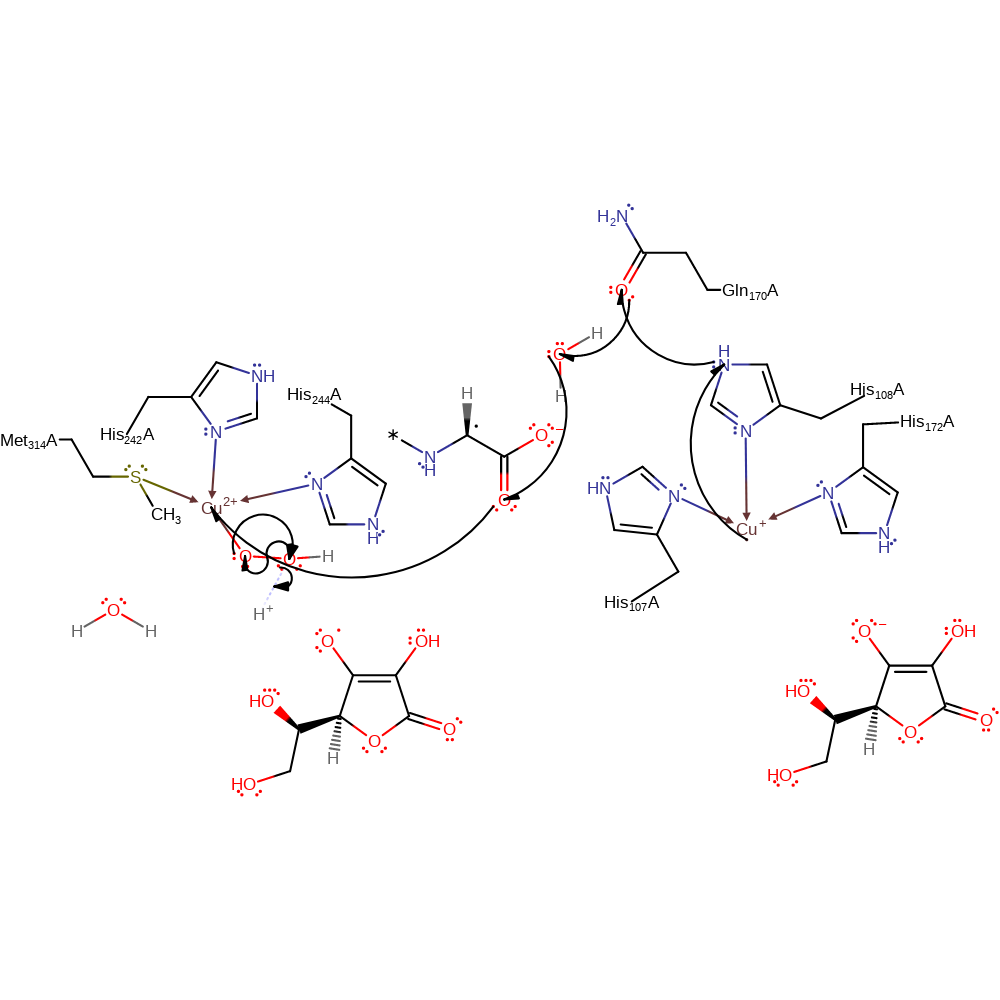
Step 5. An electron is transferred from one copper centre , Cu (I), to another, Cu (II), and in turn the Cu(II)-hyrdoperoxo is protonated resulting in the reduction of oxygen which is followed by homolysis and production of the radical species.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
| Gln170(128)A | hydrogen bond acceptor |
| His108(66)A | hydrogen bond donor, single electron relay |
| Gln170(128)A | single electron relay, single electron donor |
| His108(66)A | single electron donor, single electron acceptor |
| Gln170(128)A | single electron acceptor |
Chemical Components
proton transfer, electron relay, electron transfer, homolysis, intermediate formation, radical propagation, redox reaction, inferred reaction step
Step 6. The Cu(II)-oxyl radical species and the peptide radical can recombine to produce the Cu(II)-alkoxide intermediate/inner sphere alcohol.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
Chemical Components
colligation, electron transfer, intermediate formation, radical termination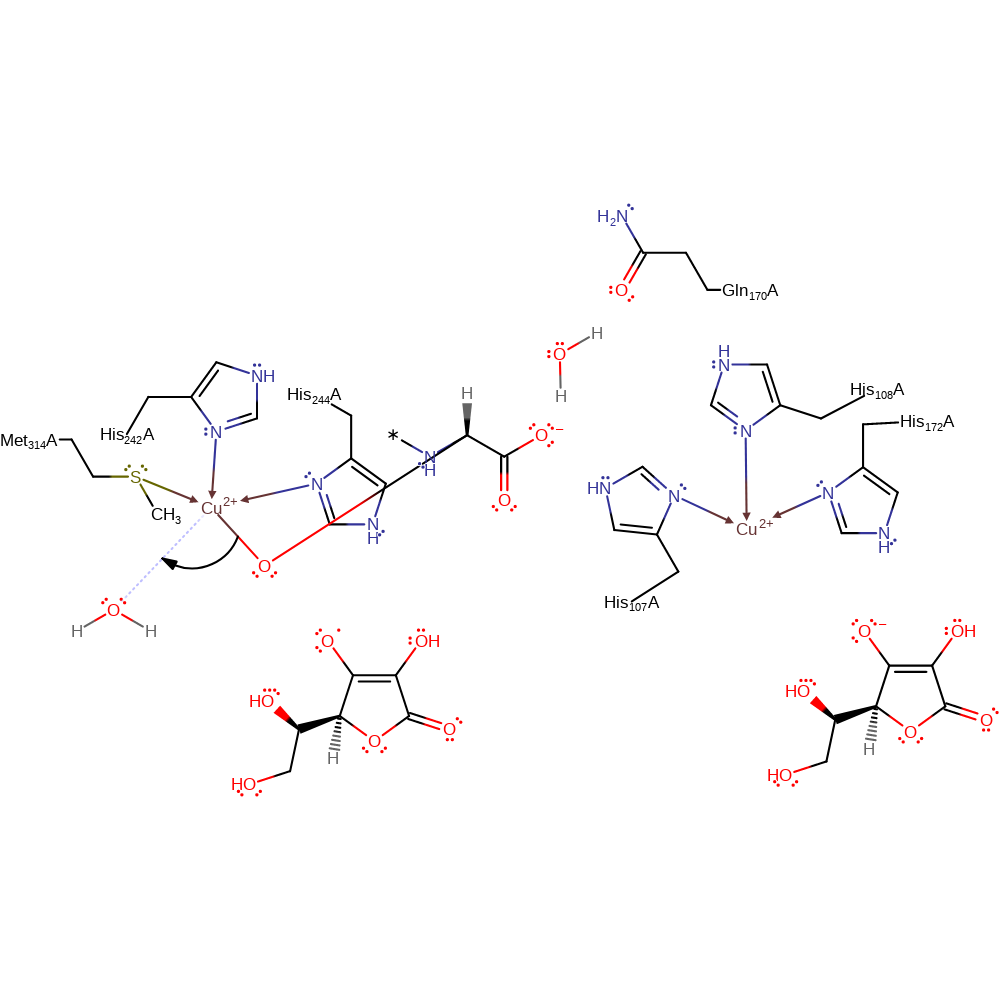
Step 7. A water molecule coordinates to Cu(II) which results in the release of the product with the protonation of the oxygen being inferred.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
Chemical Components
coordination to a metal ion, elimination (not covered by the Ingold mechanisms), intermediate collapse, intermediate terminated, overall product formed
Step 8. The two semidehydroascorbates disproportionate to yield ascorbate and dehydroascorbate
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|
Chemical Components
native state of cofactor regenerated, overall product formed, reaction occurs outside the enzyme, radical terminationIntroduction
A highly reduced copper-oxo species is proposed in this mechanism for substrate C-H bond cleavage which is preceded by electron transfer from one copper centre to another. Similarly to other mechanism the 2 Copper centres are reduced by ascorbates and one donates an electron to a dioxygen which produces the end on Cu(II)-superoxo radical species. The Cu(II)-superoxo species will be converted to the highly reduces copper-oxo species by coupling intramolecular electron transfer with the acquisition of two protons leading to water release. The reduced copper-oxo species is Cu(II)-oxyl with two unpaired electrons ferromagnetically coupled to an unpaired electron delocalized within the CuM domain yielding a quartet spin state. Here, the abstraction of a hydrogen from the substrate is concerted with the spin inversion to a doublet ground state of which allows the oxidation of the substrate and the release of the hydroxylated product.
Catalytic Residues Roles
| UniProt | PDB* (1sdw) | ||
| His108 | His108(66)A | Part if the Copper A binding site (also known as the CuM site), and part of a single electon relay chain that links the two copper sites. | single electron relay, hydrogen bond donor, metal ligand, single electron acceptor, single electron donor |
| Gln170 | Gln170(128)A | Part of the single electron relay chain that links the two copper sites. | single electron relay, single electron donor, single electron acceptor, hydrogen bond acceptor |
| His107, His172 | His107(65)A, His172(130)A | Forms part of the Copper A binding site (also known as the CuM site). | metal ligand |
| His244, Met314, His242 | His244(202)A, Met314(272)A, His242(200)A | Forms part of the Copper B binding site (also known as the CuH site). | metal ligand |
Chemical Components
redox reaction, radical formation, elimination (not covered by the Ingold mechanisms), overall reactant used, decoordination from a metal ion, intermediate formation, cofactor used, homolysis, coordination to a metal ion, bimolecular homolytic addition, electron relay, electron transfer, colligation, proton transfer, bimolecular homolytic substitution, hydrogen transfer, radical propagation, intermediate collapse, intermediate terminated, overall product formed, radical termination, native state of cofactor regenerated, reaction occurs outside the enzymeReferences
- McIntyre NR et al. (2009), J Am Chem Soc, 131, 10308-10319. Imino-oxy acetic acid dealkylation as evidence for an inner-sphere alcohol intermediate in the reaction catalyzed by peptidylglycine alpha-hydroxylating monooxygenase. DOI:10.1021/ja902716d. PMID:19569683.

Step 1. Ascorbate donates a single electron to one of the Cu(II) centres, which causes the hydroxide ligand to dissociate from the Cu(II) centre.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Met314(272)A | metal ligand |
| His244(202)A | metal ligand |
| His242(200)A | metal ligand |
| His172(130)A | metal ligand |
| His108(66)A | metal ligand |
| His107(65)A | metal ligand |
| His108(66)A | hydrogen bond donor |
| Gln170(128)A | hydrogen bond acceptor |
Chemical Components
redox reaction, radical formation, elimination (not covered by the Ingold mechanisms), overall reactant used, decoordination from a metal ion, intermediate formation, cofactor used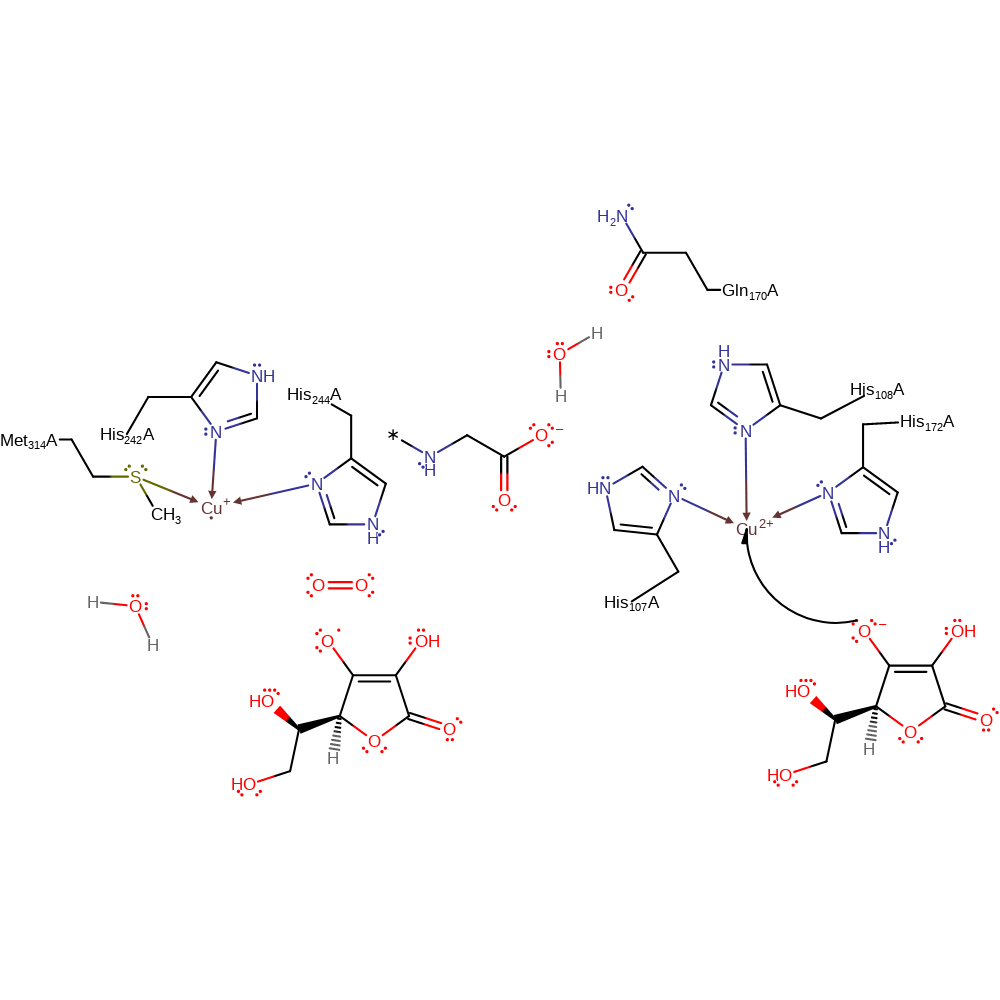
Step 2. A second ascorbate donates a single electron to the other Cu(II) centre, which causes the water ligand to dissociate from the Cu(II) centre.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Met314(272)A | metal ligand |
| His244(202)A | metal ligand |
| His242(200)A | metal ligand |
| His172(130)A | metal ligand |
| His108(66)A | metal ligand |
| His107(65)A | metal ligand |
| His108(66)A | hydrogen bond donor |
| Gln170(128)A | hydrogen bond acceptor |
Chemical Components
redox reaction, radical formation, elimination (not covered by the Ingold mechanisms), overall reactant used, decoordination from a metal ion, intermediate formation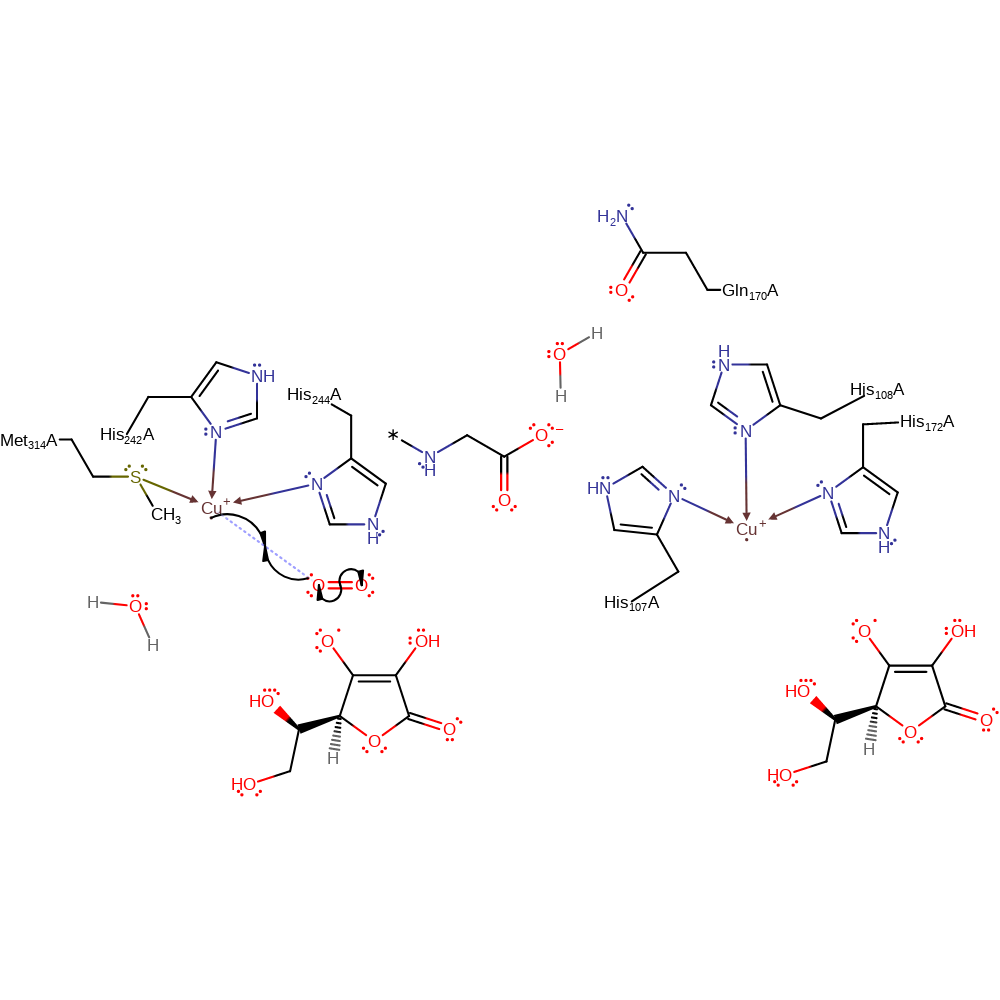
Step 3. One of the Cu(I) centres donates a single electron to a dioxygen molecule, which causes it to bind to the copper centre.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
| His108(66)A | hydrogen bond donor |
| Gln170(128)A | hydrogen bond acceptor |
Chemical Components
intermediate formation, homolysis, coordination to a metal ion, overall reactant used, radical formation, redox reaction, ingold: bimolecular homolytic addition
Step 4. A single electron relay is started from the transfer of an electron from one Cu(I) centre through His108, Gln170, water and the peptide product to the Cu(II) centre.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
| His108(66)A | hydrogen bond donor |
| Gln170(128)A | hydrogen bond acceptor |
| His108(66)A | single electron relay |
| Gln170(128)A | single electron relay, single electron donor |
| His108(66)A | single electron donor, single electron acceptor |
| Gln170(128)A | single electron acceptor |
Chemical Components
electron relay, electron transfer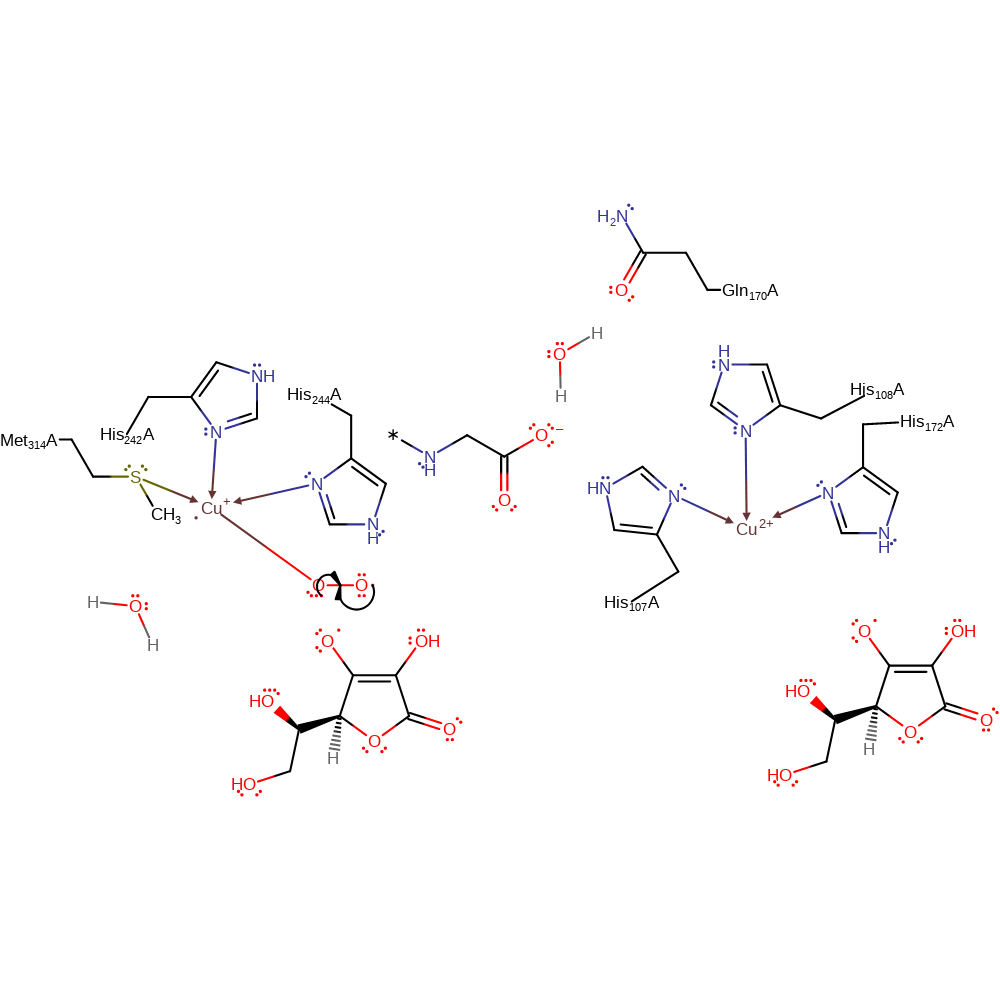
Step 5. Transfer of an electron to the copper centre is followed by the formation of a double bond between the oxygens.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
| Gln170(128)A | hydrogen bond acceptor |
| His108(66)A | hydrogen bond donor |
Chemical Components
colligation, intermediate formation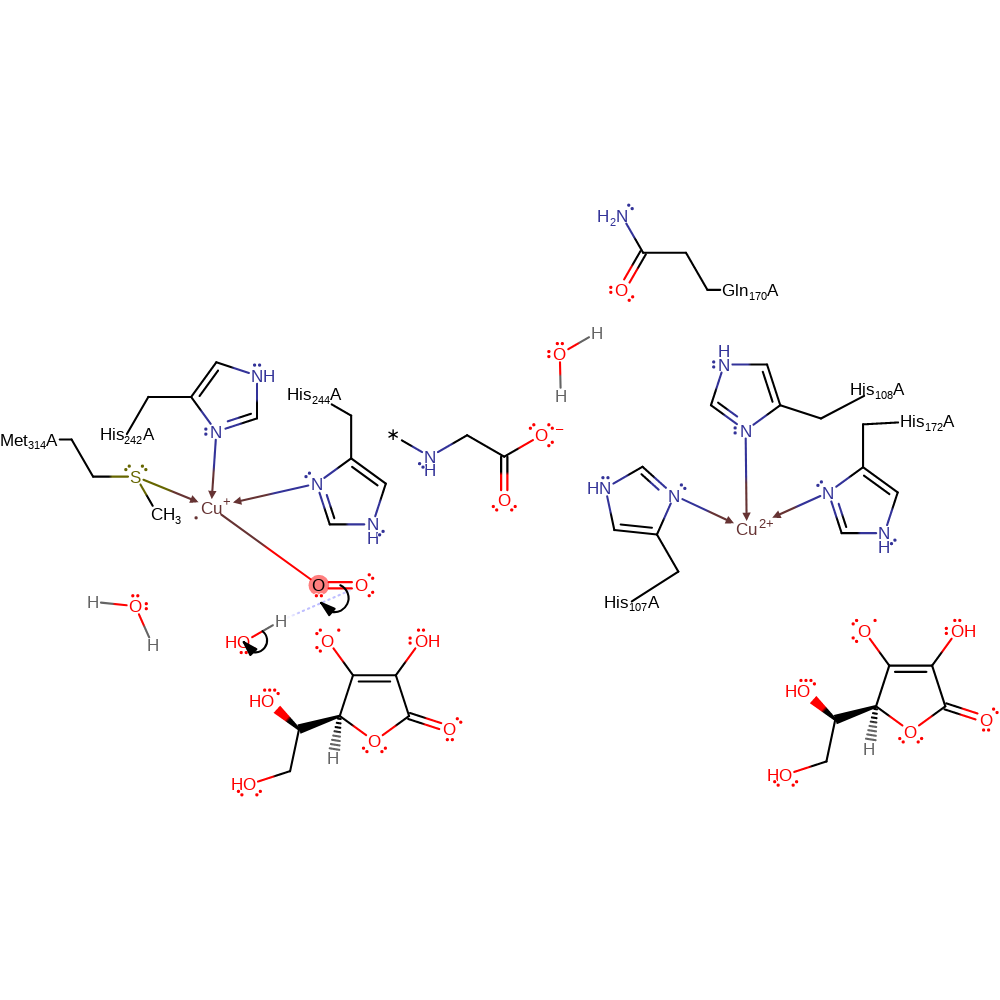
Step 6. Cu(I)-superoxo species is reduced through the acquisition of a proton.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
Chemical Components
proton transfer, intermediate formation
Step 7. A highly reduced copper-oxo species is produces from the transfer of an electron and a proton which results in the release of water as well.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
| His108(66)A | hydrogen bond donor |
| Gln170(128)A | hydrogen bond acceptor |
Chemical Components
electron transfer, proton transfer, radical formation, intermediate formation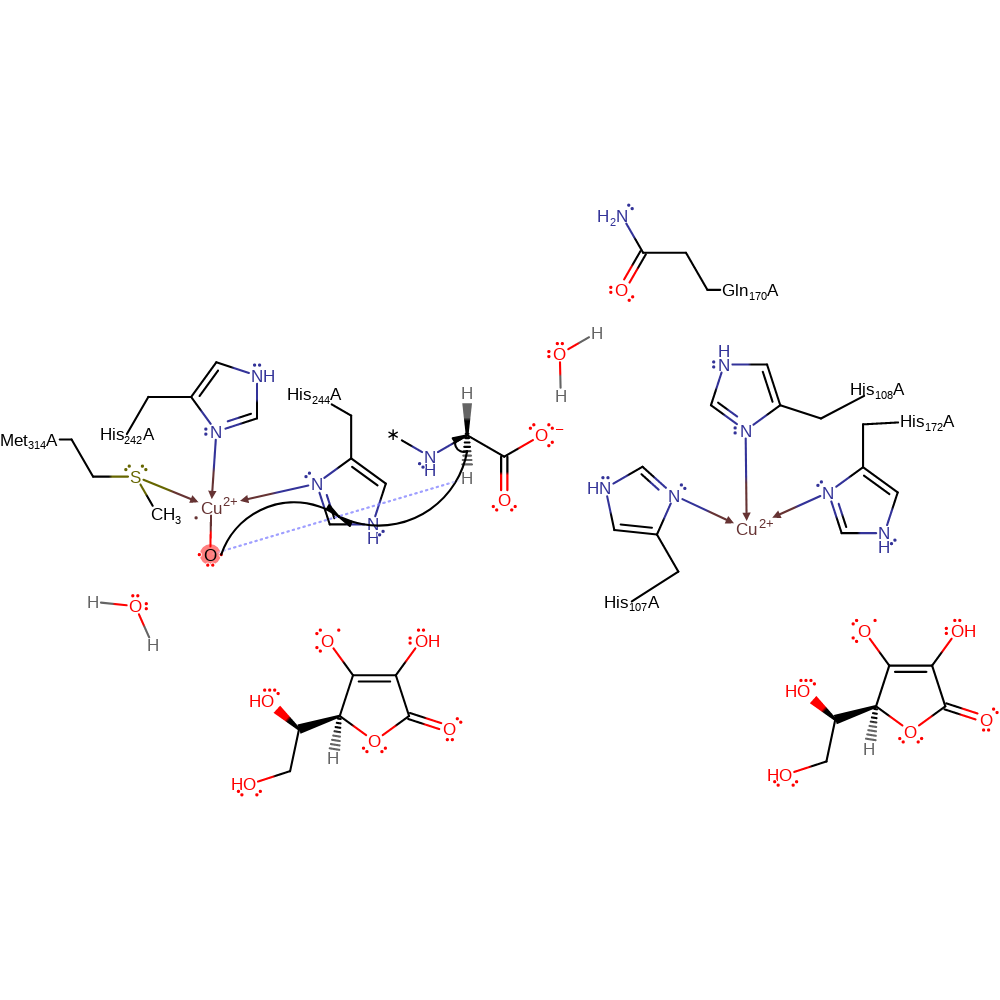
Step 8. Cu(II)-oxyl abstracts a proton from the substrate propagating the radical and this is concerted with the spin inversion to a doublet ground state.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
| His108(66)A | hydrogen bond donor |
| Gln170(128)A | hydrogen bond acceptor |
Chemical Components
ingold: bimolecular homolytic substitution, hydrogen transfer, radical propagation, overall reactant used, intermediate formation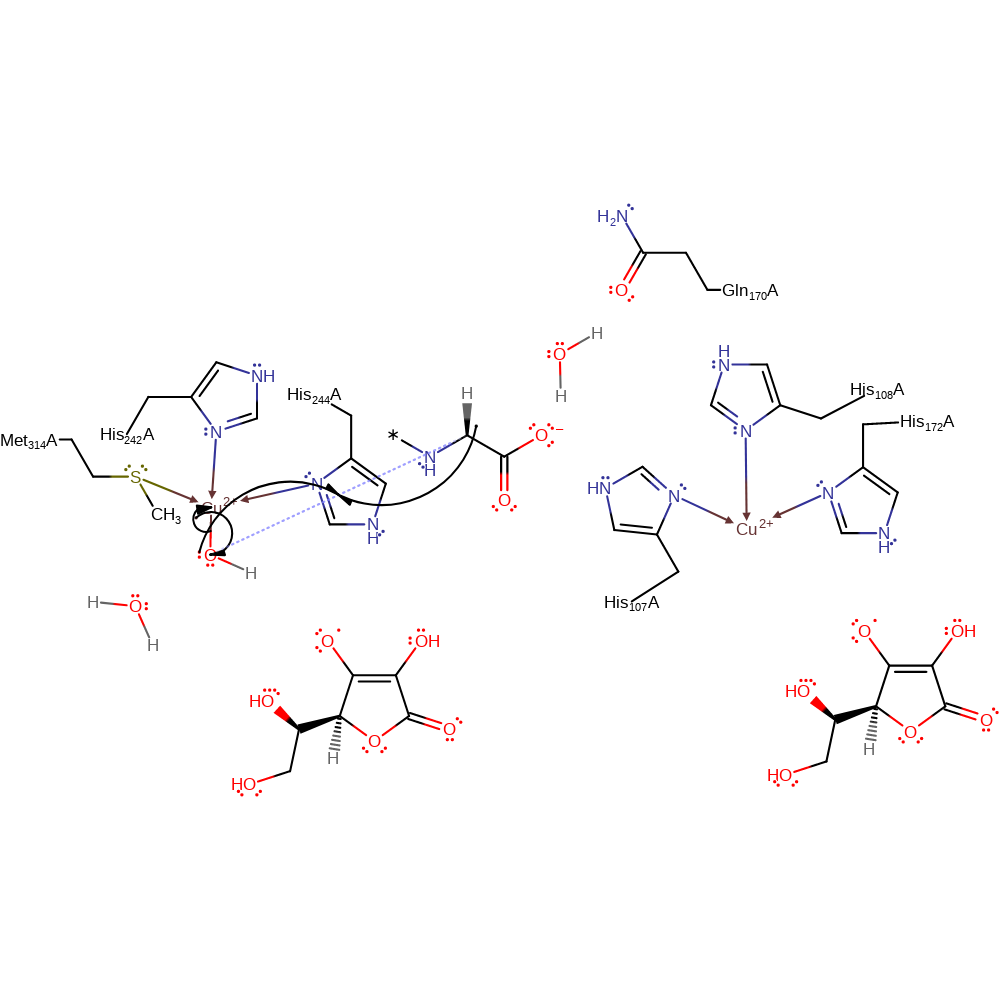
Step 9. The doublet ground state species produced allows substrate oxidation and release of the hydroxylated product which is displaced by a water molecule coordinated to Cu(II).
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
Chemical Components
ingold: bimolecular homolytic substitution, coordination to a metal ion, decoordination from a metal ion, colligation, intermediate collapse, intermediate terminated, overall product formed, radical termination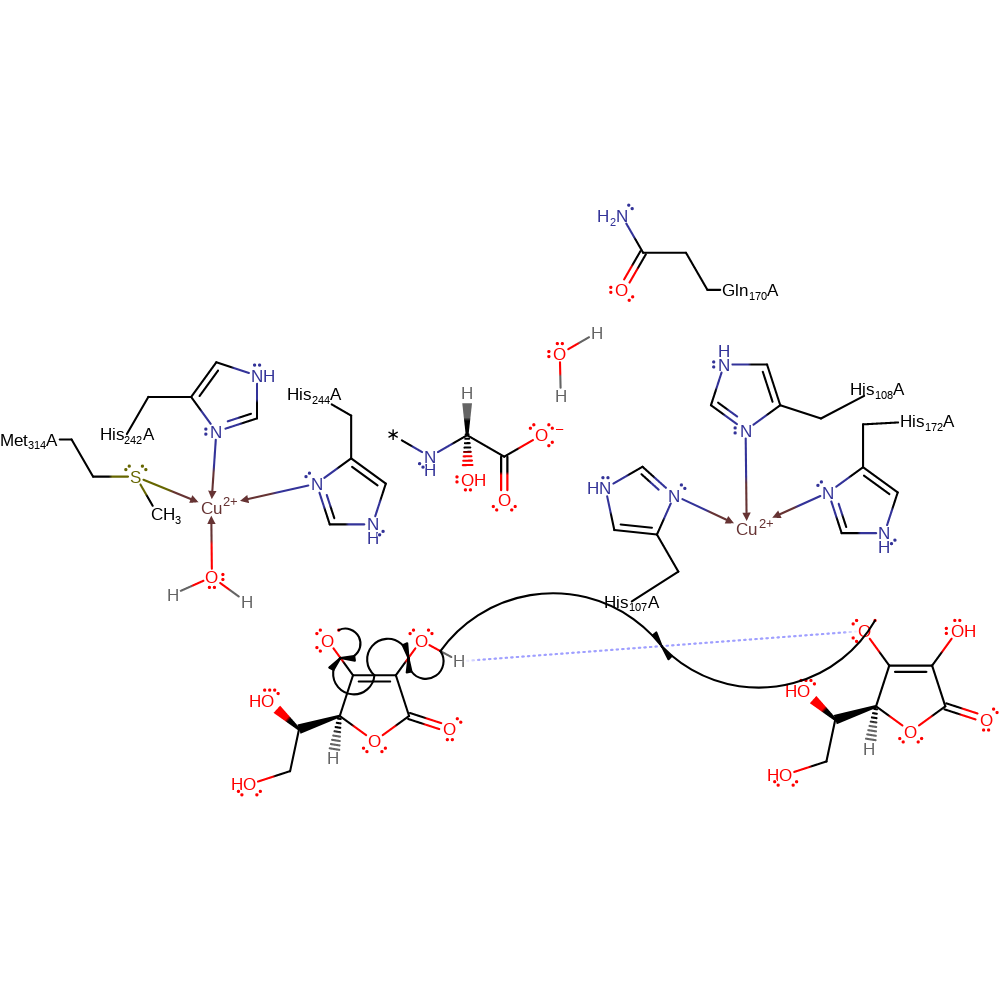
Step 10. The two semidehydroascorbates disproportionate to yield ascorbate and dehydroascorbate
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|
Chemical Components
native state of cofactor regenerated, overall product formed, reaction occurs outside the enzyme, radical terminationIntroduction
Similarly to mechanism proposal 3, A highly reduced copper-oxo species is formed for substrate C-H bond cleavage which is preceded by electron transfer from one copper centre to another. However it differs to the other proposal in that the reduced copper-oxo species formed is a triplet ground state which following a spin inversion yields an antiferromagnetically coupled singlet state to drive concerted H-abstraction with substrate oxidation/product release. This Cu(II)-oxyl species has been shown to be the more thermodynamically stable state for H-abstraction [PMID:19569683].
Catalytic Residues Roles
| UniProt | PDB* (1sdw) | ||
| His108 | His108(66)A | Part of the Copper A binding site (also known as the CuM site), and part of a single electron relay chain that links the two copper sites. | single electron relay, hydrogen bond donor, metal ligand, single electron acceptor, single electron donor |
| Gln170 | Gln170(128)A | Part of the single electron relay chain that links the two copper sites. | single electron relay, single electron donor, single electron acceptor, hydrogen bond acceptor |
| His107, His172 | His107(65)A, His172(130)A | Forms part of the Copper A binding site (also known as the CuM site). | metal ligand |
| His244, Met314, His242 | His244(202)A, Met314(272)A, His242(200)A | Forms part of the Copper B binding site (also known as the CuH site). | metal ligand |
Chemical Components
redox reaction, radical formation, elimination (not covered by the Ingold mechanisms), overall reactant used, decoordination from a metal ion, intermediate formation, cofactor used, bimolecular homolytic addition, coordination to a metal ion, homolysis, electron transfer, electron relay, colligation, proton transfer, bimolecular homolytic substitution, hydrogen transfer, radical propagation, intermediate collapse, intermediate terminated, overall product formed, radical termination, native state of cofactor regenerated, reaction occurs outside the enzymeReferences
- McIntyre NR et al. (2009), J Am Chem Soc, 131, 10308-10319. Imino-oxy acetic acid dealkylation as evidence for an inner-sphere alcohol intermediate in the reaction catalyzed by peptidylglycine alpha-hydroxylating monooxygenase. DOI:10.1021/ja902716d. PMID:19569683.
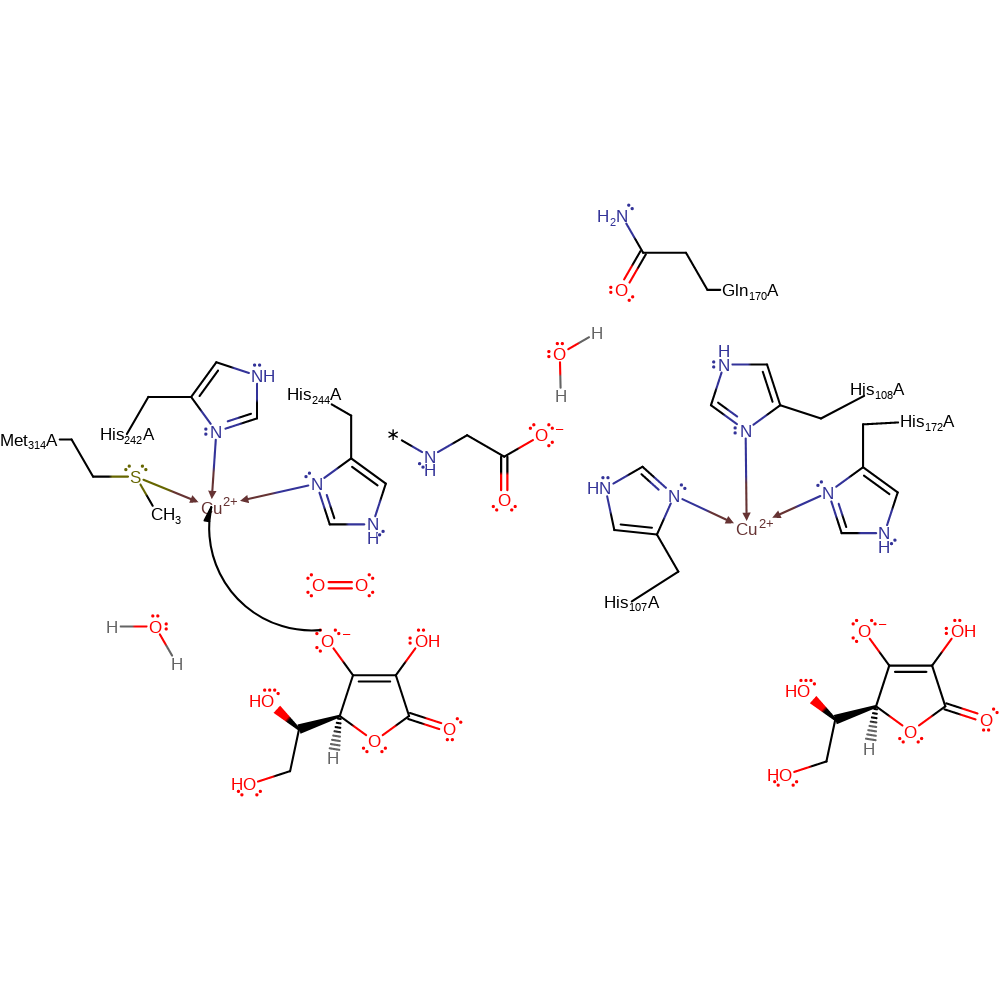
Step 1. Ascorbate donates a single electron to one of the Cu(II) centres, which causes the hydroxide ligand to dissociate from the Cu(II) centre.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Met314(272)A | metal ligand |
| His244(202)A | metal ligand |
| His242(200)A | metal ligand |
| His172(130)A | metal ligand |
| His108(66)A | metal ligand |
| His107(65)A | metal ligand |
| His108(66)A | hydrogen bond donor |
| Gln170(128)A | hydrogen bond acceptor |
Chemical Components
redox reaction, radical formation, elimination (not covered by the Ingold mechanisms), overall reactant used, decoordination from a metal ion, intermediate formation, cofactor used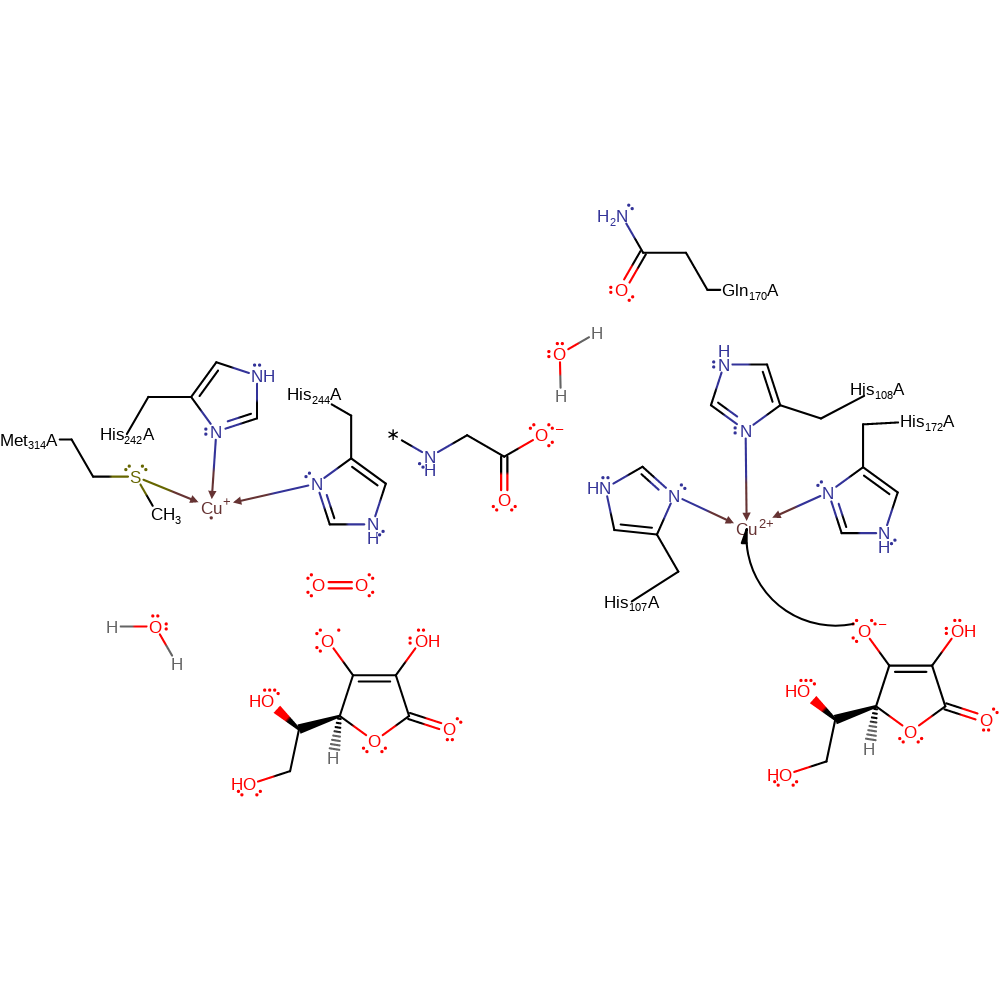
Step 2. A second ascorbate donates a single electron to the other Cu(II) centre, which causes the water ligand to dissociate from the Cu(II) centre.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Met314(272)A | metal ligand |
| His244(202)A | metal ligand |
| His242(200)A | metal ligand |
| His172(130)A | metal ligand |
| His108(66)A | metal ligand |
| His107(65)A | metal ligand |
| His108(66)A | hydrogen bond donor |
| Gln170(128)A | hydrogen bond acceptor |
Chemical Components
redox reaction, radical formation, elimination (not covered by the Ingold mechanisms), overall reactant used, decoordination from a metal ion, intermediate formation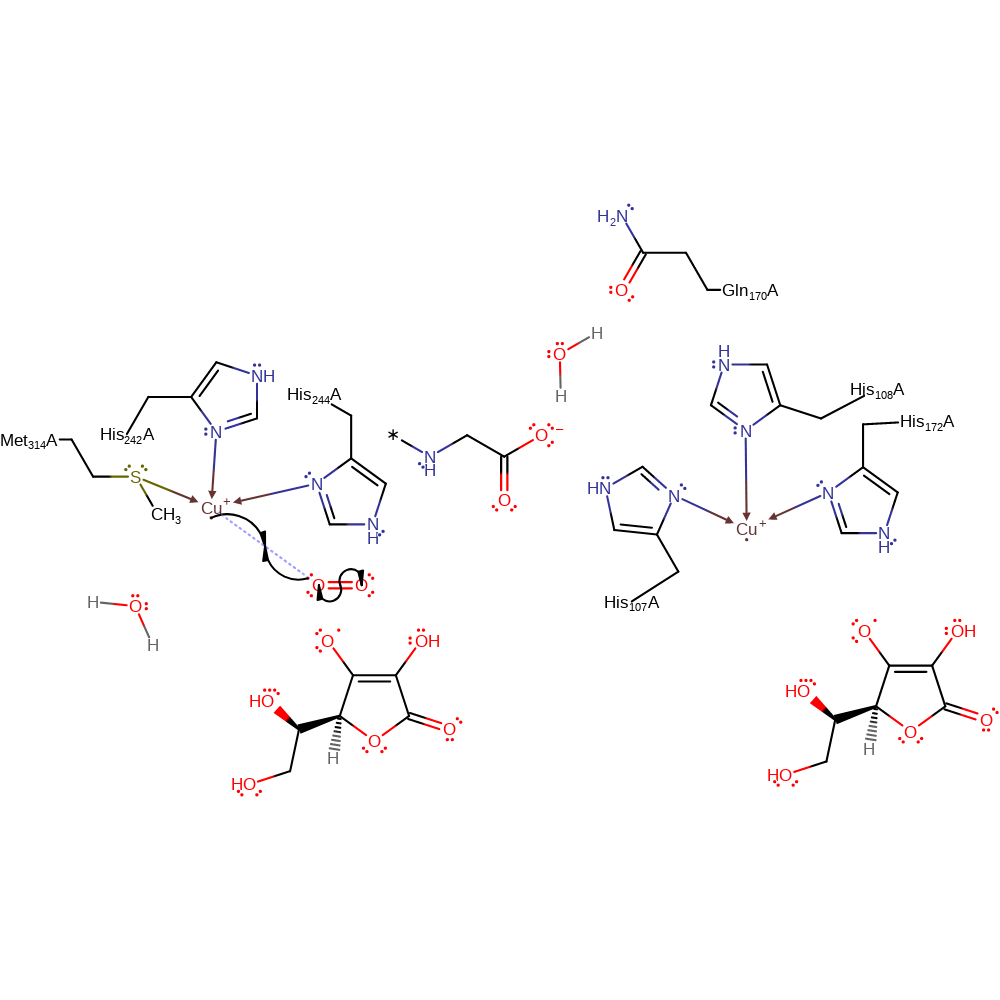
Step 3. One of the Cu(I) centres donates a single electron to a dioxygen molecule, which causes it to bind to the copper centre.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Gln170(128)A | hydrogen bond acceptor |
| His108(66)A | hydrogen bond donor |
| Met314(272)A | metal ligand |
| His244(202)A | metal ligand |
| His242(200)A | metal ligand |
| His172(130)A | metal ligand |
| His108(66)A | metal ligand |
| His107(65)A | metal ligand |
Chemical Components
ingold: bimolecular homolytic addition, redox reaction, radical formation, overall reactant used, coordination to a metal ion, homolysis, intermediate formation
Step 4. A single electron relay is started from the transfer of an electron from one Cu(I) centre through His108, Gln170, water and the peptide product to the Cu(II) centre.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Gln170(128)A | hydrogen bond acceptor |
| His108(66)A | hydrogen bond donor |
| Met314(272)A | metal ligand |
| His244(202)A | metal ligand |
| His242(200)A | metal ligand |
| His172(130)A | metal ligand |
| His108(66)A | metal ligand |
| His107(65)A | metal ligand |
| His108(66)A | single electron acceptor |
| Gln170(128)A | single electron acceptor |
| His108(66)A | single electron relay |
| Gln170(128)A | single electron relay, single electron donor |
| His108(66)A | single electron donor |
Chemical Components
electron transfer, electron relay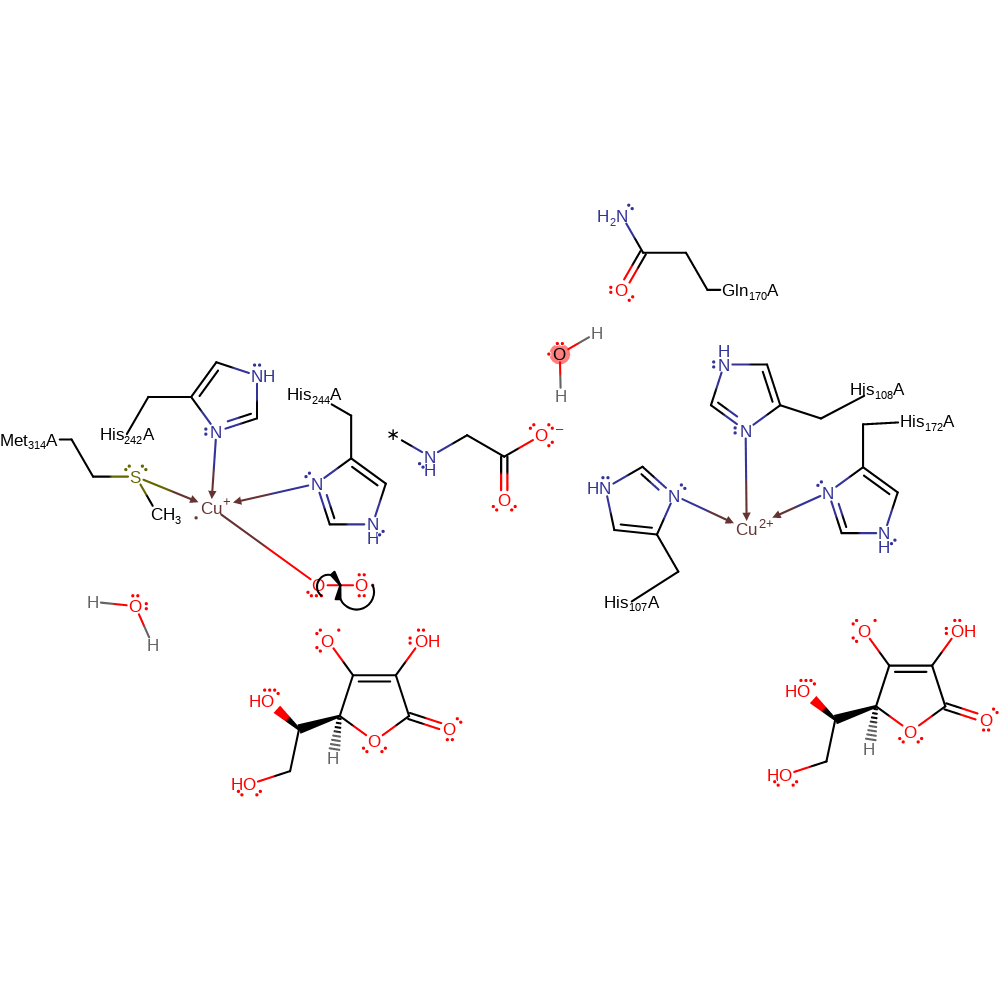
Step 5. Transfer of an electron to the copper centre is followed by the formation of a double bond between the oxygens.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His108(66)A | hydrogen bond donor |
| Gln170(128)A | hydrogen bond acceptor |
| Met314(272)A | metal ligand |
| His244(202)A | metal ligand |
| His242(200)A | metal ligand |
| His172(130)A | metal ligand |
| His108(66)A | metal ligand |
| His107(65)A | metal ligand |
Chemical Components
intermediate formation, colligation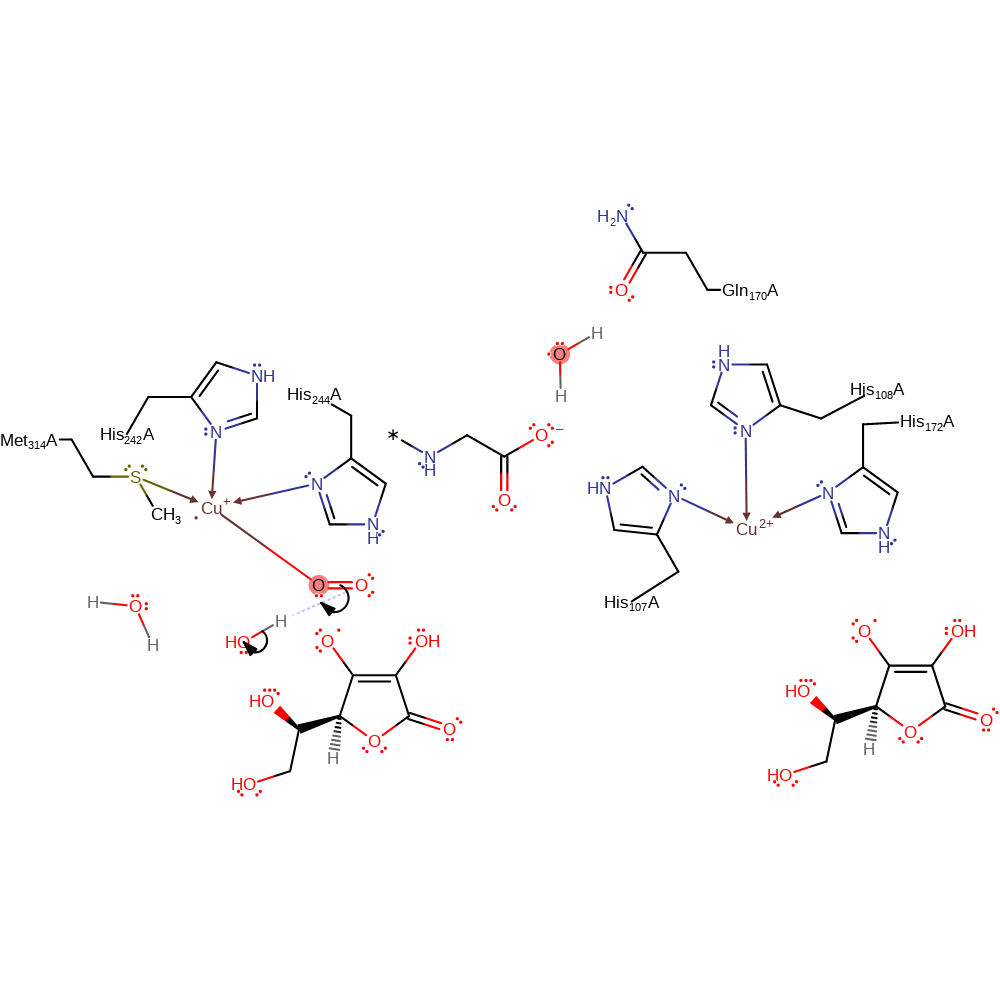
Step 6. Cu(I)-superoxo species is reduced through the acquisition of a proton.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Met314(272)A | metal ligand |
| His244(202)A | metal ligand |
| His242(200)A | metal ligand |
| His172(130)A | metal ligand |
| His108(66)A | metal ligand |
| His107(65)A | metal ligand |
Chemical Components
intermediate formation, proton transfer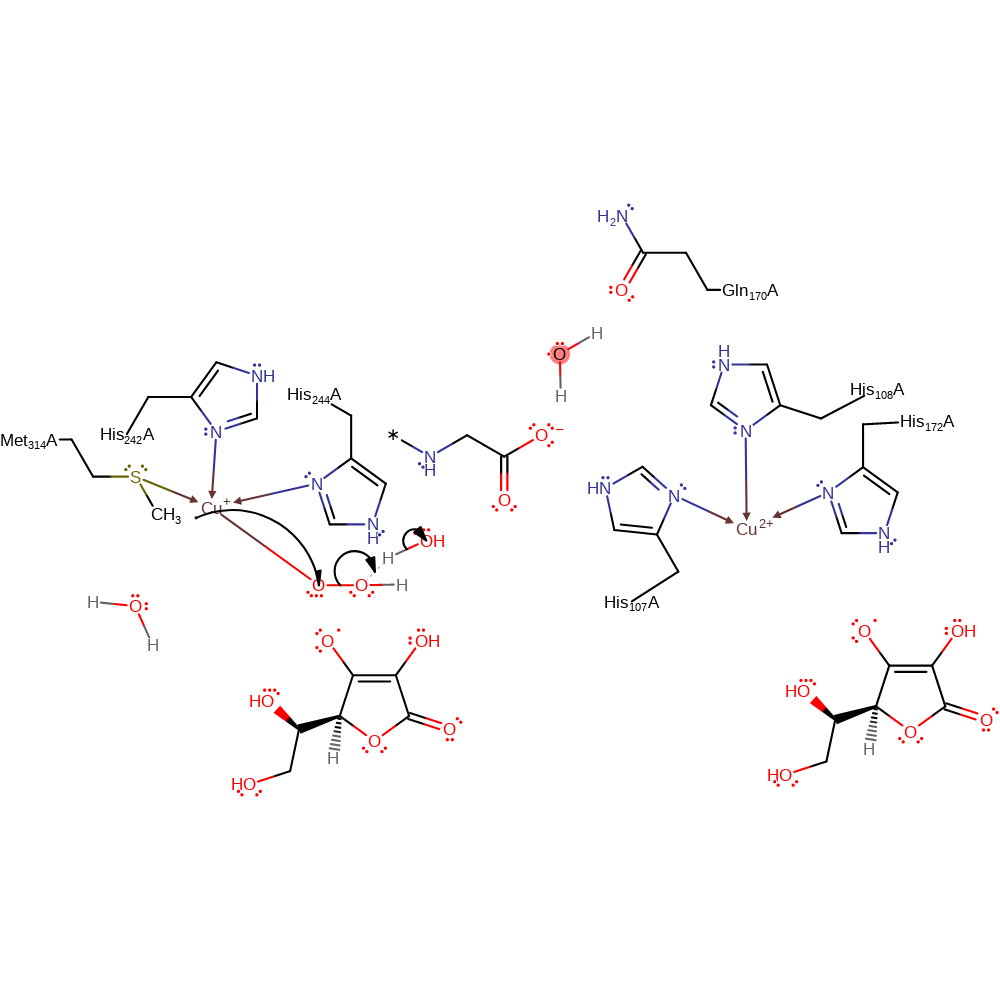
Step 7. A highly reduced copper-oxo species is produces from the transfer of an electron and a proton which results in the release of water as well.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Gln170(128)A | hydrogen bond acceptor |
| His108(66)A | hydrogen bond donor |
| Met314(272)A | metal ligand |
| His244(202)A | metal ligand |
| His242(200)A | metal ligand |
| His172(130)A | metal ligand |
| His108(66)A | metal ligand |
| His107(65)A | metal ligand |
Chemical Components
intermediate formation, radical formation, proton transfer, electron transfer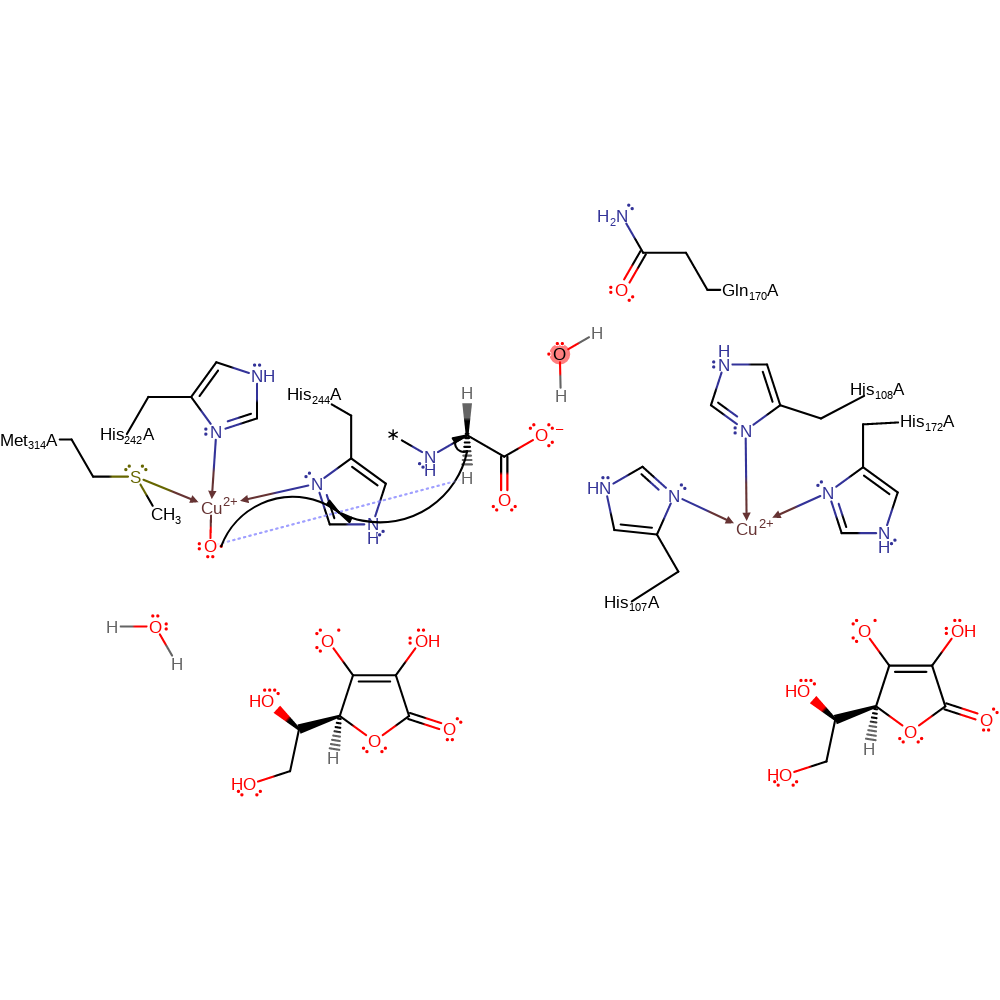
Step 8. Spin inversion yields an antiferromagnetically coupled singlet state Cu(II)-oxyl to drive H-abstraction from the substrate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
| Gln170(128)A | hydrogen bond acceptor |
| His108(66)A | hydrogen bond donor |
Chemical Components
ingold: bimolecular homolytic substitution, hydrogen transfer, radical propagation, overall reactant used, intermediate formation, colligation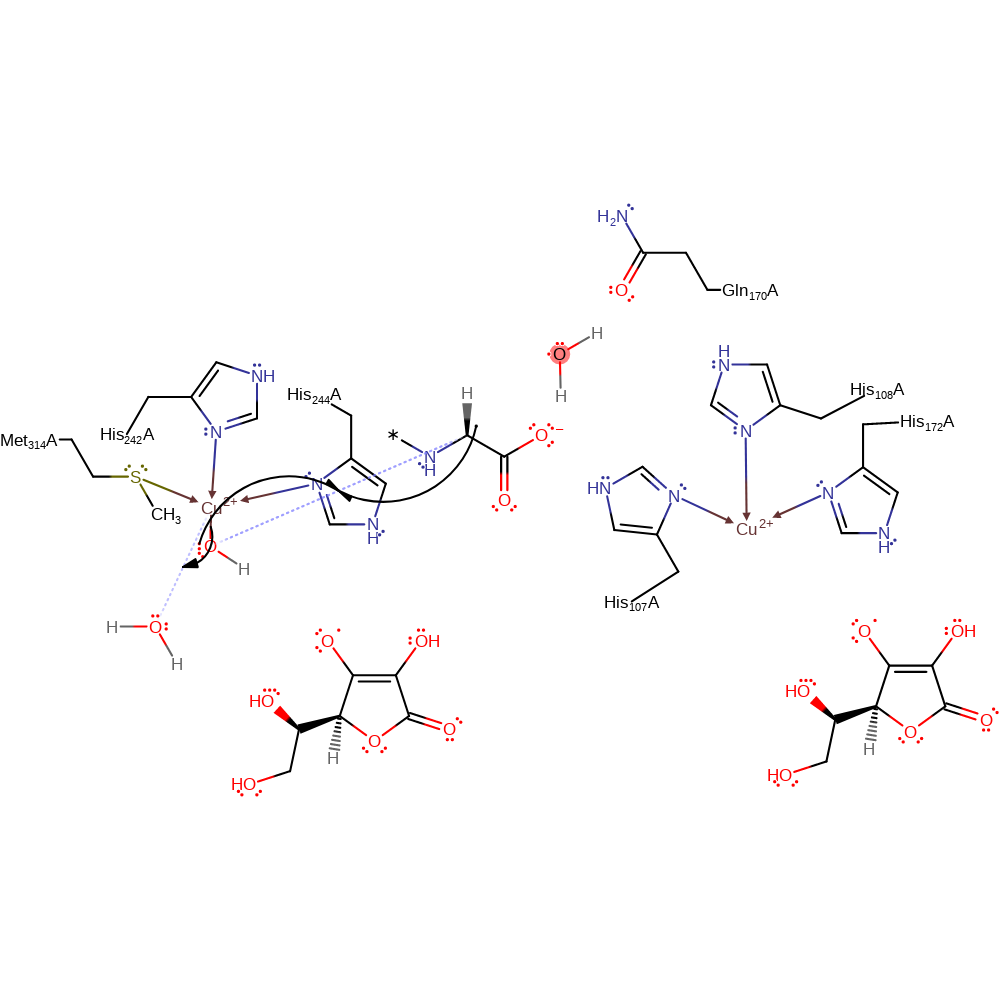
Step 9. The radical substrate is oxidised and the hydroxylated product is released by displacement by a water molecule which coordinates to Cu(II).
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His107(65)A | metal ligand |
| His108(66)A | metal ligand |
| His172(130)A | metal ligand |
| His242(200)A | metal ligand |
| His244(202)A | metal ligand |
| Met314(272)A | metal ligand |
| Gln170(128)A | hydrogen bond acceptor |
| His108(66)A | hydrogen bond donor |
Chemical Components
ingold: bimolecular homolytic substitution, coordination to a metal ion, decoordination from a metal ion, colligation, intermediate collapse, intermediate terminated, overall product formed, radical termination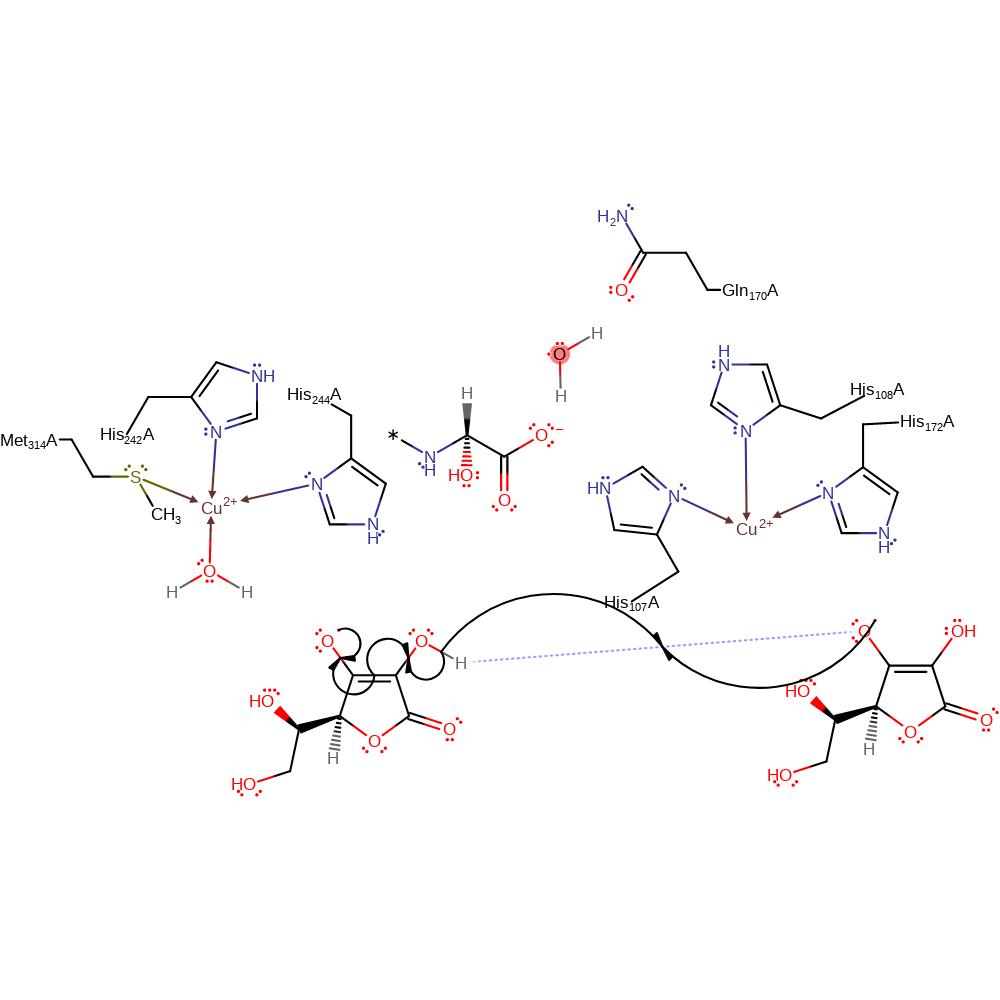
Step 10. The two semidehydroascorbates disproportionate to yield ascorbate and dehydroascorbate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|






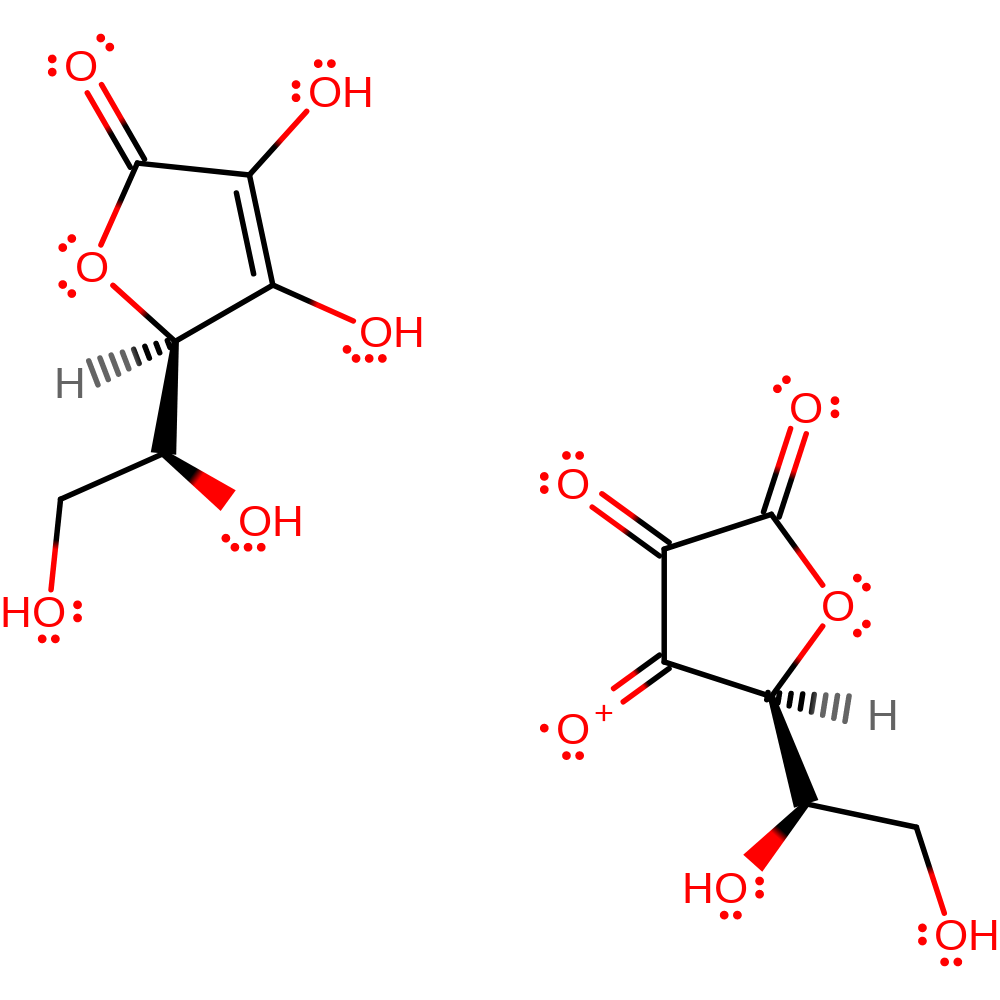 Download:
Download:  Download:
Download: 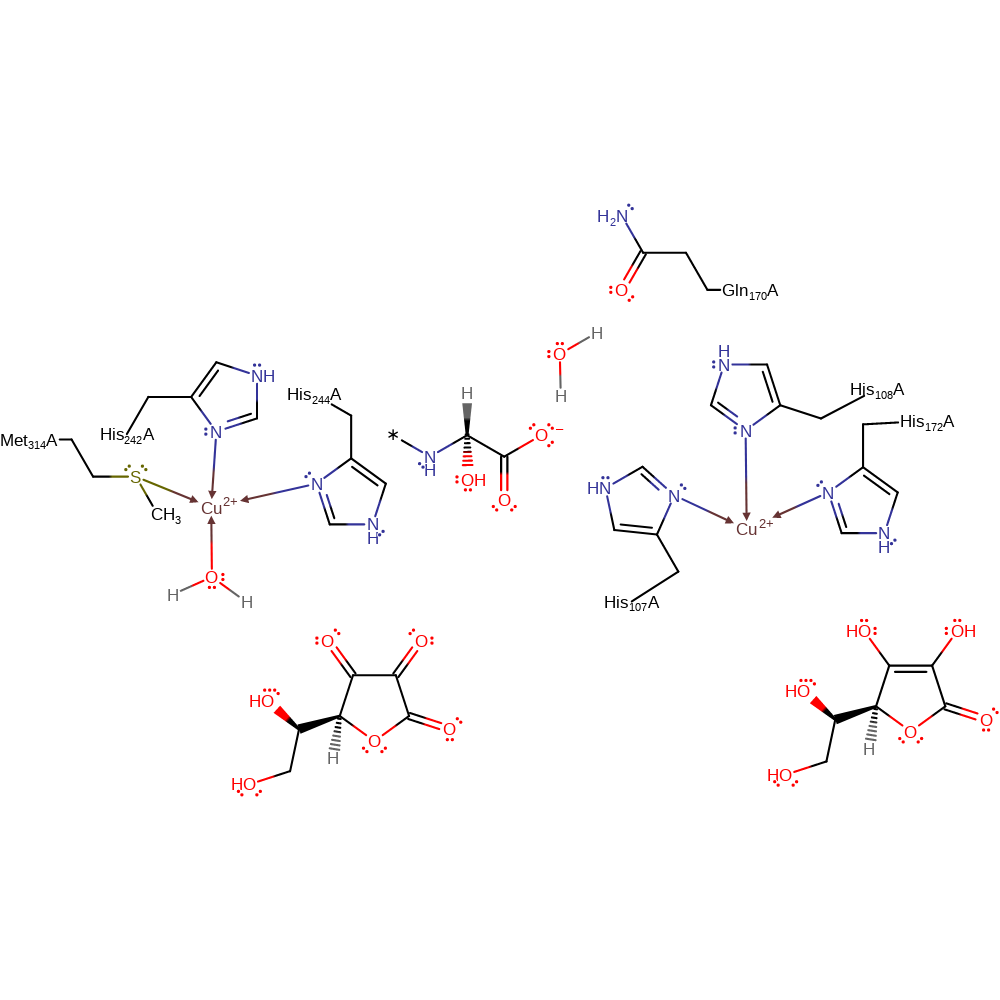 Download:
Download: 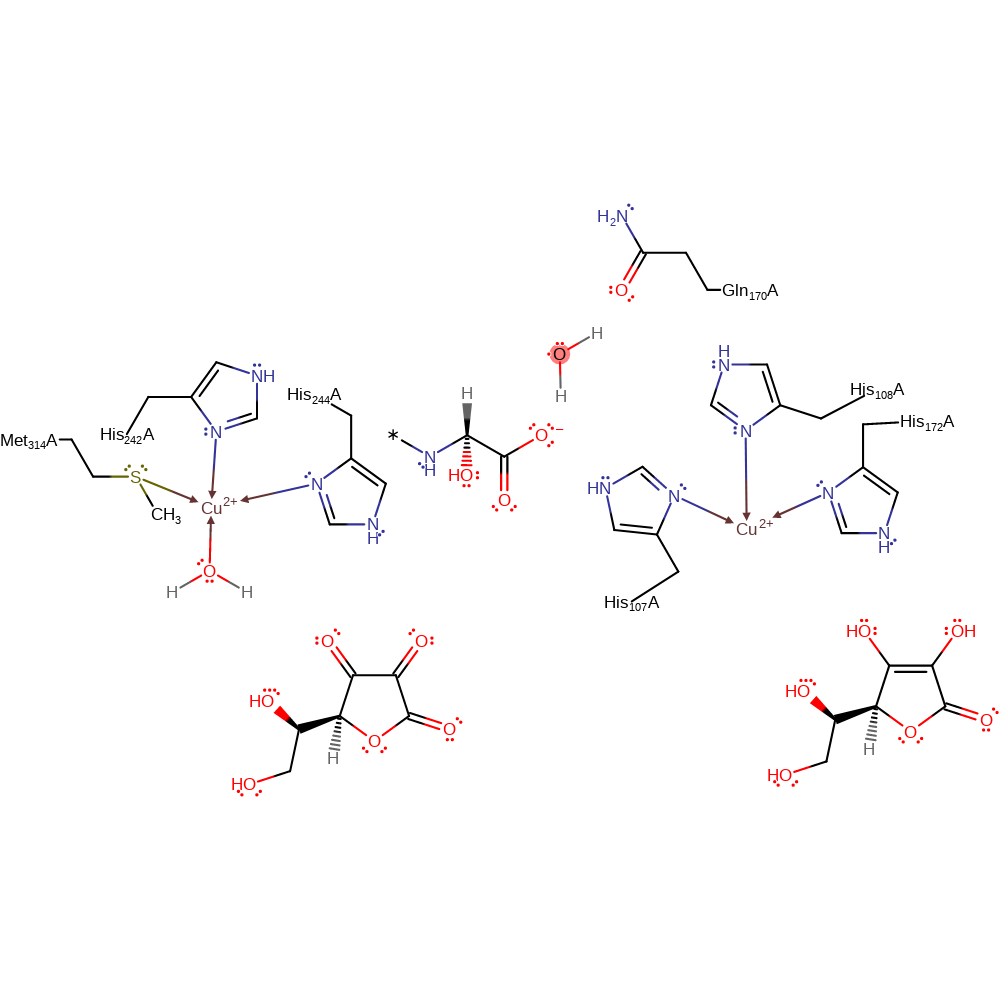 Download:
Download: