Pyruvate:ferredoxin oxidoreducatse
Pyruvate:ferrodoxin/flavodoxin reductases (PFORs) catalyse the oxidative decarboxylation of pyruvate to acetyl-CoA in anaerobic organisms. PFORs can occur in both obligately and facultatively anaerobic bacteria and also some eukaryotic microorganisms. PFORs are single-chain enzymes containing a thiamin pyrophosphate cofactor for the cleavage of carbon-carbon bonds next to a carbonyl group, and iron-sulphur clusters for electron transfer. Ferredoxin I and ferredoxin II, which are single 4Fe-4S cluster ferredoxins are the most effective electron carriers for PFORs in Desulfovibrio africanus.
Reference Protein and Structure
- Sequence
-
P94692
 (1.2.7.1)
(1.2.7.1)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Desulfovibrio africanus (Bacteria)

- PDB
-
2c3m
- Crystal Structure Of Pyruvate-Ferredoxin Oxidoreductase From Desulfovibrio africanus
(1.84 Å)



- Catalytic CATH Domains
-
3.40.50.970
 (see all for 2c3m)
(see all for 2c3m)
- Cofactors
- Tetra-mu3-sulfido-tetrairon (3), Thiamine(1+) diphosphate(3-) (1), Magnesium(2+) (1) Metal MACiE
Enzyme Reaction (EC:1.2.7.1)
Enzyme Mechanism
Introduction
The catalytic mechanism of PDC for the most part follows the principles of catalytic mechanisms of other TPP-dependent enzymes: carbonyl addition of pyruvate to the reactive C2 atom of the cofactor thiazolium ring yields the intermediate 2-(2-lactyl)-TDP (LTDP). The subsequent release of carbon dioxide produces resonating carbanion/enamine forms of 2-(1-hydroxyethyl)-TDP (HETDP, also known as hydroxyethylidene-TPP). The resonating form is considered to be a central and highly reactive intermediate state in TPP-dependent enzymes acting on pyruvate. However, unlike most other TPP-dependent enzymes in which the intermediate is oxidized, the carbanion/enamine in PDC is protonated at the C2α position, yielding C2alpha-hydroxylethylthiamine diphosphate (HETDP) before the final release of acetaldehyde completes the reaction.
Catalytic Residues Roles
| UniProt | PDB* (2c3m) | ||
| Glu64 | Glu64(63)A | Acts as the general acid/base that activates the TPP cofactor. | hydrogen bond acceptor, hydrogen bond donor, proton acceptor, proton donor |
| Asn996, Thr31, Arg114 | Asn996(995)A, Thr31(30)A, Arg114(113)A | Help activate and stabilise the reactive intermediates formed during the course of the reaction. | hydrogen bond donor, electrostatic stabiliser |
Chemical Components
proton transfer, assisted tautomerisation (not keto-enol), cofactor used, intermediate formation, bimolecular nucleophilic addition, unimolecular elimination by the conjugate base, hydride transfer, tautomerisation (not keto-enol), redox reaction, overall reactant used, native state of cofactor regenerated, decarboxylation, overall product formed, intermediate collapse, electron relay, homolysis, radical formation, colligation, intermediate terminated, native state of enzyme regenerated, inferred reaction stepReferences
- Cavazza C et al. (2006), Structure, 14, 217-224. Flexibility of Thiamine Diphosphate Revealed by Kinetic Crystallographic Studies of the Reaction of Pyruvate-Ferredoxin Oxidoreductase with Pyruvate. DOI:10.1016/j.str.2005.10.013. PMID:16472741.
- Eram MS et al. (2013), Biomolecules, 3, 578-596. Decarboxylation of Pyruvate to Acetaldehyde for Ethanol Production by Hyperthermophiles. DOI:10.3390/biom3030578. PMID:24970182.
- Reed GH et al. (2012), Biochim Biophys Acta, 1824, 1291-1298. Radical reactions of thiamin pyrophosphate in 2-oxoacid oxidoreductases. DOI:10.1016/j.bbapap.2011.11.010. PMID:22178227.
- Mansoorabadi SO et al. (2006), Biochemistry, 45, 7122-7131. EPR Spectroscopic and Computational Characterization of the Hydroxyethylidene-Thiamine Pyrophosphate Radical Intermediate of Pyruvate:Ferredoxin Oxidoreductase†. DOI:10.1021/bi0602516. PMID:16752902.
- Chabrière E et al. (2001), Science, 294, 2559-2563. Crystal Structure of the Free Radical Intermediate of Pyruvate:Ferredoxin Oxidoreductase. DOI:10.1126/science.1066198. PMID:11752578.
- Chabrière E et al. (1999), Nat Struct Biol, 6, 182-190. Crystal structures of the key anaerobic enzyme pyruvate:ferredoxin oxidoreductase, free and in complex with pyruvate. DOI:10.1038/5870. PMID:10048931.
- Pieulle L et al. (1995), Biochim Biophys Acta, 1250, 49-59. Isolation and characterization of the pyruvate-ferredoxin oxidoreductase from the sulfate-reducing bacterium Desulfovibrio africanus. PMID:7612653.
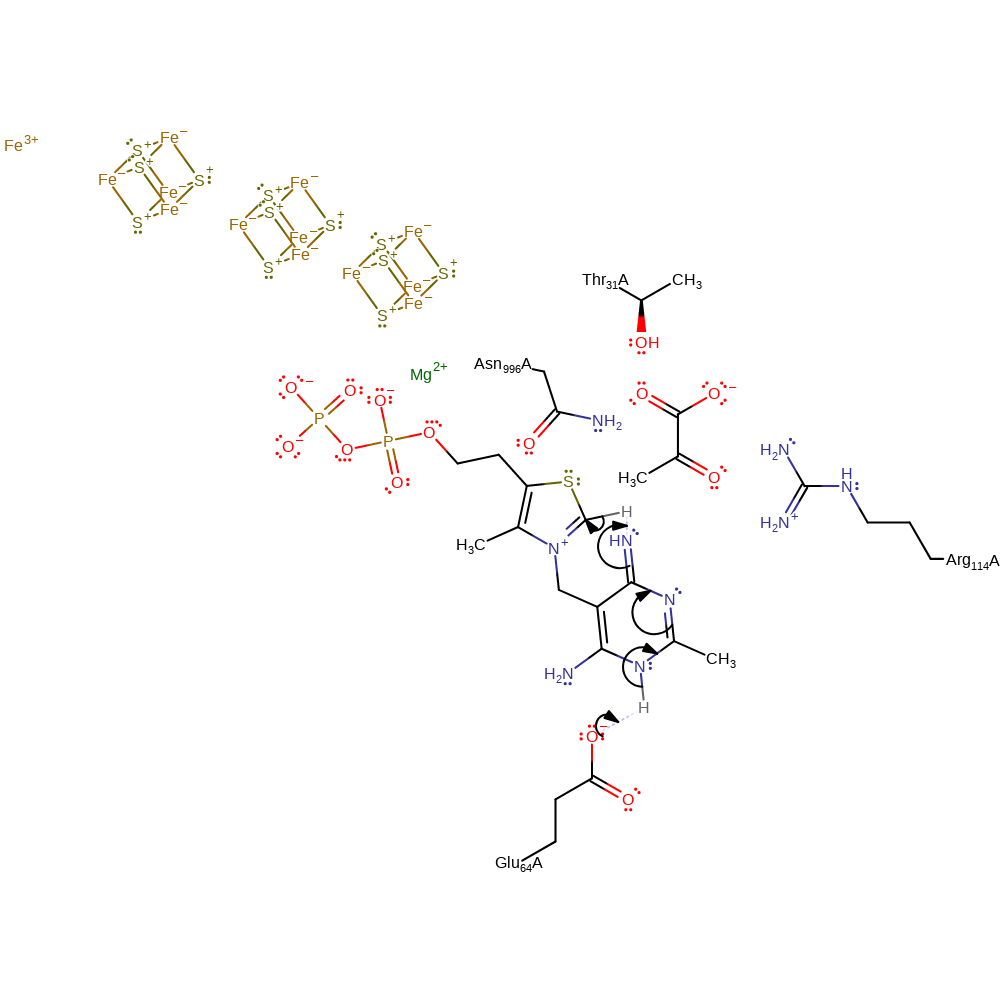
Step 1. Glu64 deprotonates the thiamine diphosphate cofactor, which initiates double bond rearrangement that results in the deprotonation of the N=CH-S group, activating the cofactor.
The cofactor adopts the V configuration that brings the 4 imino group of the aminopyrimidine ring close to the C2 carbon of the thiazolium ring [PMID:16472741]. Hydrogen bonding of the carboxylate group from Glu64 to N1' of the aminopyrimidine ring increases the basic nature of the 4' position by generating the 4'-imino tautomer [PMID:11752578]. Binding of the substrate triggers the proton transfers [PMID10048931]. The mechanism shown here is supported by crystallographic studies [PMID:16472741].
Catalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu64(63)A | hydrogen bond acceptor |
| Asn996(995)A | hydrogen bond donor, electrostatic stabiliser |
| Glu64(63)A | proton acceptor |
Chemical Components
proton transfer, assisted tautomerisation (not keto-enol), cofactor used, intermediate formation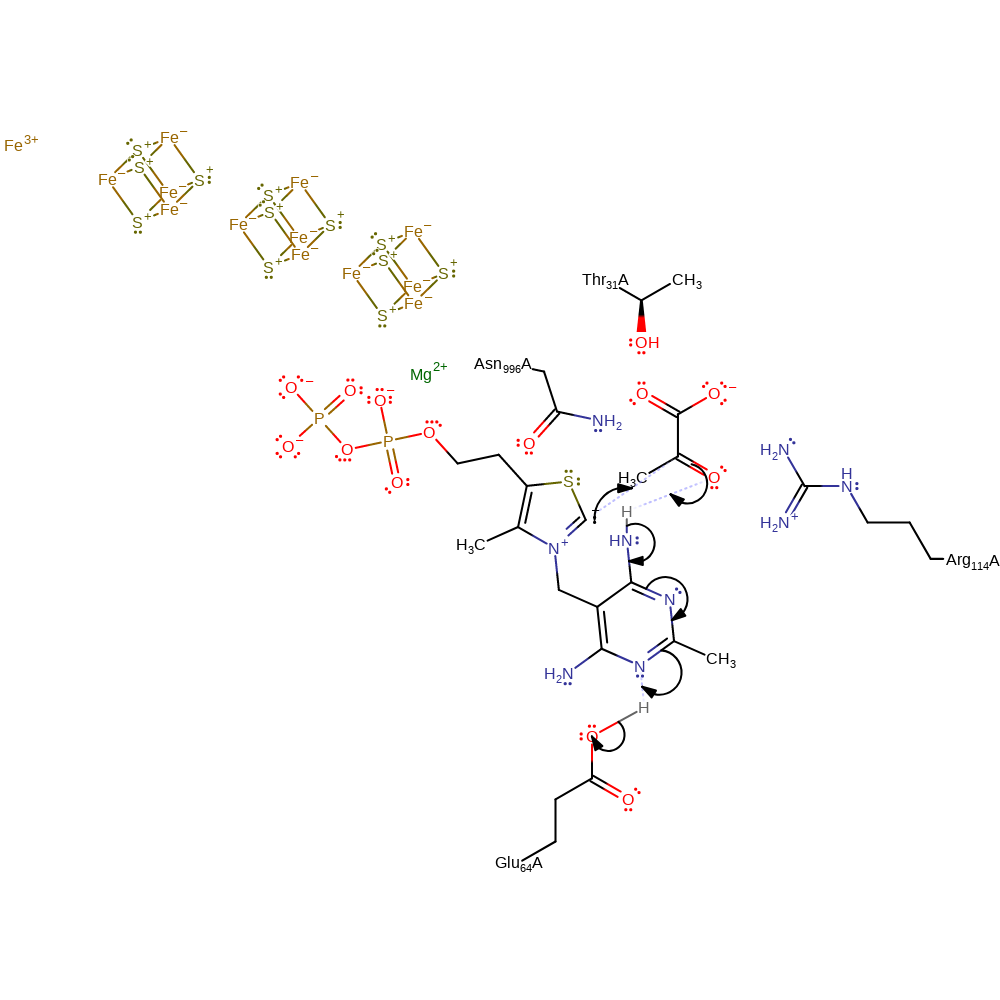
Step 2. The carbanion of thiamine diphosphate initiates a nucleophilic attack on the carbonyl carbon of pyruvate in an addition reaction that results in the cofactor undergoing double bond rearrangement that results in the deprotonation of Glu64.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn996(995)A | hydrogen bond donor, electrostatic stabiliser |
| Thr31(30)A | hydrogen bond donor, electrostatic stabiliser |
| Glu64(63)A | hydrogen bond donor |
| Arg114(113)A | hydrogen bond donor, electrostatic stabiliser |
| Glu64(63)A | proton donor |
Chemical Components
ingold: bimolecular nucleophilic addition, proton transfer, assisted tautomerisation (not keto-enol), intermediate formation
Step 3. The covalently bound pyruvate undergoes decarboxylation, resulting in double bond rearrangement, a single electron being transferred to ferredoxin via three iron-sulfur clusters and the formation of a one-electron bond between the cofactor and the formyl group.
The carbon dioxide reaction product remains tightly bound in the active site, consistent with the reversibility of the reaction [PMID:16472741]. The C2alpha-C2 bond between the acetyl and thiazole moieties is a one-electron unusually long bond PMID:11752578,PMID:16472741] and thus incompatible with enamine formation as postulated for a standard pyruvate decarboxylation cycle [PMID:16472741]. The generated intermediate shown here is supported by crystallographic studies [PMID:16472741].
Catalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn996(995)A | hydrogen bond donor, electrostatic stabiliser |
| Thr31(30)A | hydrogen bond donor, electrostatic stabiliser |
| Glu64(63)A | hydrogen bond acceptor |
| Arg114(113)A | hydrogen bond donor, electrostatic stabiliser |
Chemical Components
ingold: unimolecular elimination by the conjugate base, hydride transfer, ingold: bimolecular nucleophilic addition, tautomerisation (not keto-enol), redox reaction, overall reactant used, cofactor used, native state of cofactor regenerated, decarboxylation, overall product formed, intermediate collapse, intermediate formation, electron relay
Step 4. The C-C one electron bond between the cofator and the formyl group fragments.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn996(995)A | hydrogen bond donor, electrostatic stabiliser |
| Glu64(63)A | hydrogen bond acceptor |
| Arg114(113)A | hydrogen bond donor |
Chemical Components
homolysis, intermediate collapse, intermediate formation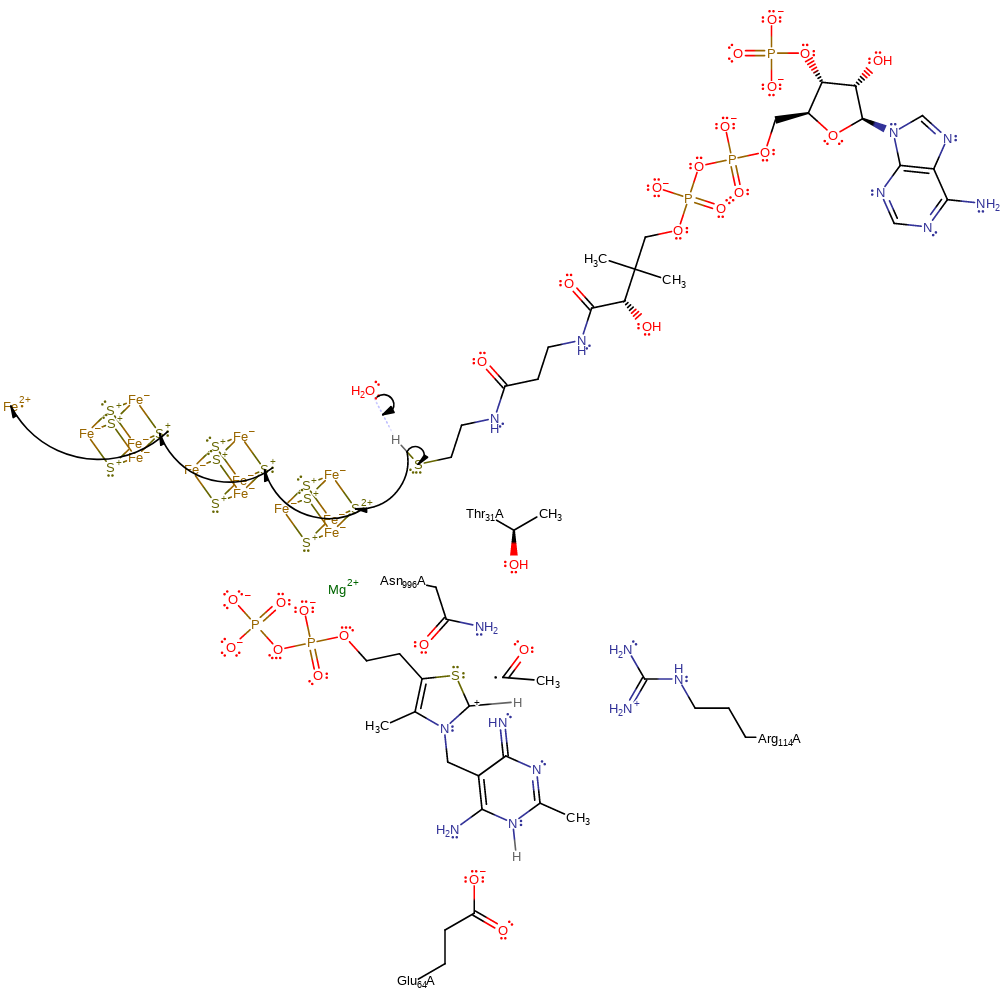
Step 5. Water deprotonates CoA, which initiates a single electron transfer to ferredoxin through three iron-sulfur clusters.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn996(995)A | hydrogen bond donor, electrostatic stabiliser |
| Glu64(63)A | hydrogen bond acceptor |
| Arg114(113)A | hydrogen bond donor |
Chemical Components
proton transfer, radical formation, redox reaction, overall reactant used, electron relay, intermediate formation, overall product formedCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn996(995)A | hydrogen bond donor, electrostatic stabiliser |
| Glu64(63)A | hydrogen bond acceptor |
| Arg114(113)A | hydrogen bond donor |
Chemical Components
colligation, intermediate terminated, overall product formed
Step 7. The cofactor is regenerated through the nitrogen donating its lone pair into the ring to satisfy the electron deficient carbon.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn996(995)A | hydrogen bond donor, electrostatic stabiliser |
| Glu64(63)A | hydrogen bond acceptor |








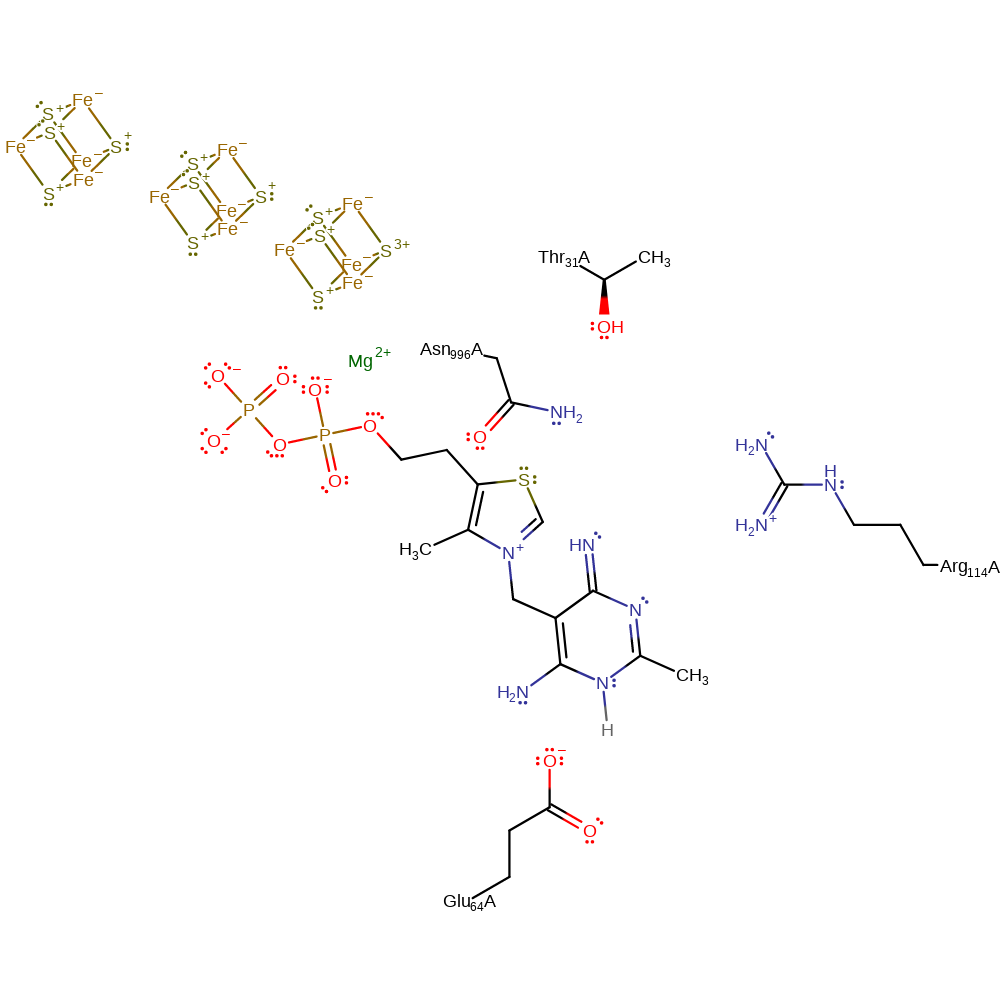 Download:
Download: