
|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
Crystal structure of the RNA polymerase i subcomplex a14/43
|
|
Structure:
|
 |
DNA-directed RNA polymerase i subunit rpa4. Chain: a, c, e. Synonym: DNA-directed DNA-dependent RNA polymerase 36 kda polypeptide, a43. Engineered: yes. DNA-directed RNA polymerase i subunit rpa4. Chain: b, d, f. Synonym: DNA-directed RNA polymerase i 14 kda polypeptide, a14. Engineered: yes
|
|
Source:
|
 |
Saccharomyces cerevisiae. Baker's yeast. Organism_taxid: 4932. Gene: rpa43, rrn12. Expressed in: escherichia coli. Expression_system_taxid: 469008.
|
|
Resolution:
|
 |
|
3.10Å
|
R-factor:
|
0.252
|
R-free:
|
0.285
|
|
|
Authors:
|
 |
S.R.Geiger,C.D.Kuhn,P.Cramer
|
Key ref:
|
 |
C.D.Kuhn
et al.
(2007).
Functional architecture of RNA polymerase I.
Cell,
131,
1260-1272.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
28-Sep-07
|
Release date:
|
15-Jan-08
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B, C, D, E, F:
E.C.2.7.7.6
- DNA-directed Rna polymerase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
RNA(n) + a ribonucleoside 5'-triphosphate = RNA(n+1) + diphosphate
|
 |
 |
 |
 |
 |
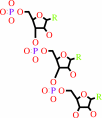 RNA(n)
RNA(n)
|
+
|
ribonucleoside 5'-triphosphate
|
=
|
RNA(n+1)
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Cell
131:1260-1272
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
Functional architecture of RNA polymerase I.
|
|
C.D.Kuhn,
S.R.Geiger,
S.Baumli,
M.Gartmann,
J.Gerber,
S.Jennebach,
T.Mielke,
H.Tschochner,
R.Beckmann,
P.Cramer.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Synthesis of ribosomal RNA (rRNA) by RNA polymerase (Pol) I is the first step in
ribosome biogenesis and a regulatory switch in eukaryotic cell growth. Here we
report the 12 A cryo-electron microscopic structure for the complete 14-subunit
yeast Pol I, a homology model for the core enzyme, and the crystal structure of
the subcomplex A14/43. In the resulting hybrid structure of Pol I, A14/43, the
clamp, and the dock domain contribute to a unique surface interacting with
promoter-specific initiation factors. The Pol I-specific subunits A49 and A34.5
form a heterodimer near the enzyme funnel that acts as a built-in elongation
factor and is related to the Pol II-associated factor TFIIF. In contrast to Pol
II, Pol I has a strong intrinsic 3'-RNA cleavage activity, which requires the
C-terminal domain of subunit A12.2 and, apparently, enables ribosomal RNA
proofreading and 3'-end trimming.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Figure 2. Model and EM Features of the Pol I Core
(A)
Placement of the Pol II ten-subunit core structure (Armache et
al., 2005) (gray) into the EM density (blue). The foot was
deleted, and subunits Rpb5, Rpb8, and Rpb9 are highlighted in
magenta, green, and orange, respectively. The clamp has been
fitted as a separate rigid body.
(B) Fit of the common
subunits Rpb5 and Rpb8 to the EM map, and density for the core
subunit A12.2 (the Pol II homolog Rpb9 is shown as a ribbon
model).
(C) Pol II structure-guided sequence alignment of
the five Pol I subunits with homologs in Pol II (compare Table
1). The domain organization of Pol II subunits Rpb1, Rpb2, Rpb3,
Rpb11, and Rpb9 is shown as diagrams (Cramer et al., 2001).
Insertions and deletions exceeding five amino acid residues are
indicated. Conserved folds are indicated by orange highlighting
of the bar above the diagrams. For details see Figure S1.
(D) View of the core Pol II structure (Cramer et al., 2001) from
the side, with domains depicted in (E) highlighted.
(E) Pol
I-specific structural elements. Fitted Pol II elements are shown
as ribbon models. Insertions and deletions explaining the EM
density are named according to (C). The clamp head is in light
red and the clamp core in red. The dock and foot domains are in
beige and blue, respectively, and Rpb3, Rpb10, and Rpb11 are in
red, dark blue, and in yellow, respectively. Zinc ions are
depicted as marine spheres.
|
 |
Figure 5.
Figure 5. Intrinsic RNA Cleavage Activity and Functional
Architecture of Pol I
(A) DNA-RNA hybrid scaffold used in
cleavage assays.
(B) Comparison of RNA cleavage by Pol I
variants with Pol II and the Pol II-TFIIS complex. Pol I mainly
removed four nucleotides from the RNA, consistent with binding
of the terminal hybrid base pair to the nucleotide insertion
site (+1), extrusion of the RNA 3′ overhang into the
polymerase pore and cleavage of the phosphodiester bond between
nucleotides at positions −1 and +1 (Figure 5A). The Pol
II-TFIIS complex removed three or four nucleotides, indicating
that a mixture of complexes was present with the terminal hybrid
base pair occupying either position −1 or +1.
(C) pH
dependence of pol I cleavage activity.
(D) Elongation
activity of the Pol I variant A12.2ΔC.
(E) Hybrid
structure and functional architecture of Pol I. The EM envelope
is shown as a blue line, the Pol I core ribbon model in gray
with Rpb9 (A12.2) highlighted in orange, and the A14/43 crystal
structure in red/blue. The window shows a cut-away view of the
active center containing a modeled DNA-RNA hybrid. Red dashes
indicate the RNA 3′ end extruded into the pore.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Cell Press:
Cell
(2007,
131,
1260-1272)
copyright 2007.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.Y.Park,
and
C.V.Robinson
(2011).
Protein-nucleic acid complexes and the role of mass spectrometry in their structure determination.
|
| |
Crit Rev Biochem Mol Biol,
46,
152-164.
|
 |
|
|
|
|
 |
B.Albert,
I.Léger-Silvestre,
C.Normand,
M.K.Ostermaier,
J.Pérez-Fernández,
K.I.Panov,
J.C.Zomerdijk,
P.Schultz,
and
O.Gadal
(2011).
RNA polymerase I-specific subunits promote polymerase clustering to enhance the rRNA gene transcription cycle.
|
| |
J Cell Biol,
192,
277-293.
|
 |
|
|
|
|
 |
L.A.Lane,
C.Fernández-Tornero,
M.Zhou,
N.Morgner,
D.Ptchelkine,
U.Steuerwald,
A.Politis,
D.Lindner,
J.Gvozdenovic,
A.C.Gavin,
C.W.Müller,
and
C.V.Robinson
(2011).
Mass spectrometry reveals stable modules in holo and apo RNA polymerases I and III.
|
| |
Structure,
19,
90.
|
 |
|
|
|
|
 |
M.Wojtas,
B.Peralta,
M.Ondiviela,
M.Mogni,
S.D.Bell,
and
N.G.Abrescia
(2011).
Archaeal RNA polymerase: the influence of the protruding stalk in crystal packing and preliminary biophysical analysis of the Rpo13 subunit.
|
| |
Biochem Soc Trans,
39,
25-30.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
P.Braglia,
J.Kawauchi,
and
N.J.Proudfoot
(2011).
Co-transcriptional RNA cleavage provides a failsafe termination mechanism for yeast RNA polymerase I.
|
| |
Nucleic Acids Res,
39,
1439-1448.
|
 |
|
|
|
|
 |
S.H.Jun,
M.J.Reichlen,
M.Tajiri,
and
K.S.Murakami
(2011).
Archaeal RNA polymerase and transcription regulation.
|
| |
Crit Rev Biochem Mol Biol,
46,
27-40.
|
 |
|
|
|
|
 |
S.Lefèvre,
H.Dumay-Odelot,
L.El-Ayoubi,
A.Budd,
P.Legrand,
N.Pinaud,
M.Teichmann,
and
S.Fribourg
(2011).
Structure-function analysis of hRPC62 provides insights into RNA polymerase III transcription initiation.
|
| |
Nat Struct Mol Biol,
18,
352-358.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.El Hage,
S.L.French,
A.L.Beyer,
and
D.Tollervey
(2010).
Loss of Topoisomerase I leads to R-loop-mediated transcriptional blocks during ribosomal RNA synthesis.
|
| |
Genes Dev,
24,
1546-1558.
|
 |
|
|
|
|
 |
C.Fernández-Tornero,
B.Böttcher,
U.J.Rashid,
U.Steuerwald,
B.Flörchinger,
D.P.Devos,
D.Lindner,
and
C.W.Müller
(2010).
Conformational flexibility of RNA polymerase III during transcriptional elongation.
|
| |
EMBO J,
29,
3762-3772.
|
 |
|
|
|
|
 |
G.A.Kassavetis,
P.Prakash,
and
E.Shim
(2010).
The C53/C37 subcomplex of RNA polymerase III lies near the active site and participates in promoter opening.
|
| |
J Biol Chem,
285,
2695-2706.
|
 |
|
|
|
|
 |
R.Carter,
and
G.Drouin
(2010).
The increase in the number of subunits in eukaryotic RNA polymerase III relative to RNA polymerase II is due to the permanent recruitment of general transcription factors.
|
| |
Mol Biol Evol,
27,
1035-1043.
|
 |
|
|
|
|
 |
S.R.Geiger,
K.Lorenzen,
A.Schreieck,
P.Hanecker,
D.Kostrewa,
A.J.Heck,
and
P.Cramer
(2010).
RNA polymerase I contains a TFIIF-related DNA-binding subcomplex.
|
| |
Mol Cell,
39,
583-594.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Hirata,
and
K.S.Murakami
(2009).
Archaeal RNA polymerase.
|
| |
Curr Opin Struct Biol,
19,
724-731.
|
 |
|
|
|
|
 |
C.Walmacq,
M.L.Kireeva,
J.Irvin,
Y.Nedialkov,
L.Lubkowska,
F.Malagon,
J.N.Strathern,
and
M.Kashlev
(2009).
Rpb9 Subunit Controls Transcription Fidelity by Delaying NTP Sequestration in RNA Polymerase II.
|
| |
J Biol Chem,
284,
19601-19612.
|
 |
|
|
|
|
 |
J.F.Sydow,
F.Brueckner,
A.C.Cheung,
G.E.Damsma,
S.Dengl,
E.Lehmann,
D.Vassylyev,
and
P.Cramer
(2009).
Structural basis of transcription: mismatch-specific fidelity mechanisms and paused RNA polymerase II with frayed RNA.
|
| |
Mol Cell,
34,
710-721.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Hirata,
T.Kanai,
T.J.Santangelo,
M.Tajiri,
K.Manabe,
J.N.Reeve,
T.Imanaka,
and
K.S.Murakami
(2008).
Archaeal RNA polymerase subunits E and F are not required for transcription in vitro, but a Thermococcus kodakarensis mutant lacking subunit F is temperature-sensitive.
|
| |
Mol Microbiol,
70,
623-633.
|
 |
|
|
|
|
 |
F.Beckouet,
S.Labarre-Mariotte,
B.Albert,
Y.Imazawa,
M.Werner,
O.Gadal,
Y.Nogi,
and
P.Thuriaux
(2008).
Two RNA polymerase I subunits control the binding and release of Rrn3 during transcription.
|
| |
Mol Cell Biol,
28,
1596-1605.
|
 |
|
|
|
|
 |
F.Werner
(2008).
Structural evolution of multisubunit RNA polymerases.
|
| |
Trends Microbiol,
16,
247-250.
|
 |
|
|
|
|
 |
H.Bierhoff,
M.Dundr,
A.A.Michels,
and
I.Grummt
(2008).
Phosphorylation by casein kinase 2 facilitates rRNA gene transcription by promoting dissociation of TIF-IA from elongating RNA polymerase I.
|
| |
Mol Cell Biol,
28,
4988-4998.
|
 |
|
|
|
|
 |
J.Kawauchi,
H.Mischo,
P.Braglia,
A.Rondon,
and
N.J.Proudfoot
(2008).
Budding yeast RNA polymerases I and II employ parallel mechanisms of transcriptional termination.
|
| |
Genes Dev,
22,
1082-1092.
|
 |
|
|
|
|
 |
K.U.Wendt,
M.S.Weiss,
P.Cramer,
and
D.W.Heinz
(2008).
Structures and diseases.
|
| |
Nat Struct Mol Biol,
15,
117-120.
|
 |
|
|
|
|
 |
P.Cramer,
K.J.Armache,
S.Baumli,
S.Benkert,
F.Brueckner,
C.Buchen,
G.E.Damsma,
S.Dengl,
S.R.Geiger,
A.J.Jasiak,
A.Jawhari,
S.Jennebach,
T.Kamenski,
H.Kettenberger,
C.D.Kuhn,
E.Lehmann,
K.Leike,
J.F.Sydow,
and
A.Vannini
(2008).
Structure of eukaryotic RNA polymerases.
|
| |
Annu Rev Biophys,
37,
337-352.
|
 |
|
|
|
|
 |
R.D.Hannan,
and
M.C.Schultz
(2008).
The 'Odd Pols' are even when it comes to controlling cell function. Conference on RNA Polymerases I and III.
|
| |
EMBO Rep,
9,
1188-1192.
|
 |
|
|
|
|
 |
S.R.Geiger,
C.D.Kuhn,
C.Leidig,
J.Renkawitz,
and
P.Cramer
(2008).
Crystallization of RNA polymerase I subcomplex A14/A43 by iterative prediction, probing and removal of flexible regions.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
64,
413-418.
|
 |
|
|
|
|
 |
Y.Ghavi-Helm,
M.Michaut,
J.Acker,
J.C.Aude,
P.Thuriaux,
M.Werner,
and
J.Soutourina
(2008).
Genome-wide location analysis reveals a role of TFIIS in RNA polymerase III transcription.
|
| |
Genes Dev,
22,
1934-1947.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

